C-C cross-coupling reactions of aryl chloride with olefinic boronate
SyntheticPage 942
Submitted: October 30, 2020, published: November 24, 2020
Authors
Shuhei Katsuta (shuhei.katsuta@tcichemicals.com)
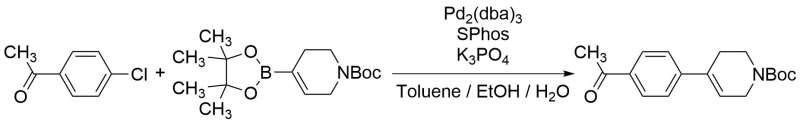
Chemicals
4'-Chloroacetophenone (TCI-C0033, as-received)
1-Boc-1,2,3,6-tetrahydropyridine-4-boronic Acid Pinacol Ester (TCI-B4051, as-received)
Tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) (TCI-T2184, as-received)
2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl (SPhos) (TCI-D5036, as-received)
K3PO4
Toluene
Ethanol
H2O
Brine
Sodium Sulfate
Ethyl acetate
Hexane
SIlica-gel for chromatography
Procedure
Under nitrogen flow, 4'-Chloroacetophenone (200 mg, 1.29 mmol) and 1-Boc-1,2,3,6-tetrahydropyridine-4-boronic acid pinacol ester (440 mg, 1.42 mmol) were dissolved in a mixture of toluene (5.0 mL), EtOH (2.0 mL) and H2O (2.0 mL). To this solution was added K3PO4 (820 mg, 3.88 mmol), 2-dicyclohexylphosphino-2',6'-dimethoxybiphenyl (SPhos) (21 mg, 0.052 mmol, 4 mol%) and tris(dibenzylideneacetone)dipalladium(0) (18 mg, 0.020 mmol, 1.5mol%). The reaction mixture was refluxed for 21 h under nitrogen flow. Then allowed to cool to room temperature and quenched with H2O (2.0 ml). The organic phase was separated and washed with H2O (5 mL) and brine (5 mL), dried over Na2SO4 (10 g), and concentrated under reduced pressure to afford the crude product as orange oil. It was purified by column chromatography on silica-gel (Hexane 100% to Hexane: EtOAc = 70: 30) to afford the product as colorless crystalline solid (310 mg, 1.03 mmol, 80%).
Author Comments
All chemical manipulations were performed in a fume hood.
Before adding the reagents, mixture of toluene, EtOH and water was degassed by refluxing for 10 minute under nitrogen flow then cooled to room temperature.
This procedure is typical for Suzuki-Miyaura cross coupling reaction of Aryl chlorides.
Pd2(dba)3-SPhos can also be adapted to the Suzuki-Miyaura cross-coupling reaction of aryl chlorides having electron-donating or bulky substitutents.
Before adding the reagents, mixture of toluene, EtOH and water was degassed by refluxing for 10 minute under nitrogen flow then cooled to room temperature.
This procedure is typical for Suzuki-Miyaura cross coupling reaction of Aryl chlorides.
Pd2(dba)3-SPhos can also be adapted to the Suzuki-Miyaura cross-coupling reaction of aryl chlorides having electron-donating or bulky substitutents.
Data
1H NMR (400 MHz, CDCl3) δ 7.90 (d, J = 8.7 Hz, 2H), 7.42 (d, J = 8.7 Hz, 2H), 6.15 (brs, 1H), 4.08 (m, 2H), 3.63 (m, 2H), 2.50 (s, 3H), 2.52 (m, 2H), 1.47 (s, 9H).
Other References
Same compound was synthesized from Aryl bromide.
(1) Kim, Ronald M.; et al, Preparation of pyrazolecarboxylic acid derivatives as soluble guanylate cyclase activators, U. S. Patent, 20100216764 A1 2010-08-26
(1) Kim, Ronald M.; et al, Preparation of pyrazolecarboxylic acid derivatives as soluble guanylate cyclase activators, U. S. Patent, 20100216764 A1 2010-08-26
Supplementary Information
Keywords
alkenes, carbocyclic compounds, coupling, transition metal catalysed