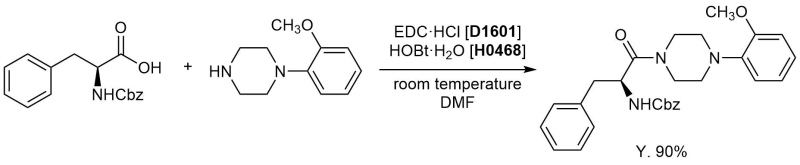
Condensation of piperidine with N-protected phenylalanine
SyntheticPage 941
Submitted: October 30, 2020, published: November 9, 2020
Authors
Shuhei Katsuta (shuhei.katsuta@tcichemicals.com)
Chemicals
N-Benzyloxycarbonyl-L-phenylalanine (TCI-C0660, as-received)
1-(2-Methoxyphenyl)piperazine (TCI-M0883, as-received)
1-Hydroxybenzotriazole Monohydrate (= HOBt·H2O) (TCI-H0468, as-received)
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride [Coupling Agent for Peptides Synthesis] (EDC·HCl) (TCI-D1601, as-received)
DMF
Ethyl acetate
Hexane
Water
Brine
Sodium Sulfate
SIlica-gel for chromatography
Hexane
Water
Brine
Sodium Sulfate
SIlica-gel for chromatography
Procedure
1-(2-methoxyphenyl)piperazine (500 mg, 2.60 mmol) was dissolved in DMF (15 mL). To this solution was added N-benzyloxycarbonyl-L-phenylalanine (770 mg, 2.60 mmol) and 1-hydroxybenzotriazole monohydrate (414 mg, 2.71 mmol). The reaction mixture was cooled to 0 °C, then 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (515 mg, 2.71 mmol) was added. Reaction was allowed to warm to room temperature, then stirred for 20 h at room temperature. Complete of the reaction was confirmed by TLC. Reaction was quenched by adding H2O (20 mL). Reaction mixture was extracted by EtOAc (30 mL). Organic phase was washed with H2O (20 mL, twice) and brine (20 mL), dried over Na2SO4, concentrated under reduced pressure to afford the crude product (1.2 g) as pale yellow oil. Crude product was purified by column chromatography on silica-gel (EtOAc: Hexane = 60: 40) to afford the product as white powder (1.10 g, 2.32 mmol, 90%).
Author Comments
All chemical manipulations were performed in a fume hood.
The reaction mixture was monitored by TLC (hexane / ethyl acetate = 4:6, Rf = 0.40)
The reaction mixture was monitored by TLC (hexane / ethyl acetate = 4:6, Rf = 0.40)
Data
1H NMR (400 MHz, CD2Cl2) δ 7.39-7.16 (m, 10H), 6.99 (m, 1H), 6.91-6.84 (m, 2H), 6.78 (m, 1H), 5.73 (d, J = 8,2 Hz, 1H), 5.12-5.01 (m, 2H), 4.91 (dd, J = 7.7, 7.3 Hz, 1H), 3.82 (s, 3H), 3.71-3.63 (m, 2H), 3.51 (m, 1H), 3.31 (m, 1H), 3.07-2.78 (m, 5H), 2.51 (m, 1H).
Lead Reference
e.g. S. Caddick, K. Aboutayab, K. Jenkins, R. I. West, J. Chem. Soc. Perkin Trans. 1, 1996, 675. DOI: 10.1039/P19960000675
Keywords
amides, amines, amino acids, carboxylic acids, dehydration condensation