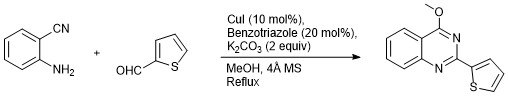
Synthesis of 4-Methoxy-2-(thiophen-2-yl)quinazoline by Cu-Benzotriazole Catalyzed Electrophilic Intramolecular Cyclization of N-Arylimine in Methanol
SyntheticPage 940
Submitted: October 27, 2020, published: January 11, 2021
Authors
Bhavyesh Desai (bhavyeshdesai42@gmail.com)
Satyanarayana Battula (satyamssd@gmail.com)
A contribution from
Chemicals
(1) 2-Aminobenzonitrile (Sigma Aldrich), 99%, 294098
(2) Thiophene-2-carbaldehyade (Sigma Aldrich), 98%, T32409
(3) Copper iodide (Sigma Aldrich), 98%, 205540
(4) Benzotriazole (Sigma Aldrich), 99%, B11400
(5) Potassium carbonate (Sigma Aldrich), 99%, 209619
(6) Molecular sieves (CDH), 054004
(7) Anhydrous sodium sulfate (RANKEM), 99%, S0410
(8) Methanol (RANKEM), directly used without drying
(9) n-hexane (SD Fine Chemicals Ltd), directly used without drying
(10) Ethyl acetate (SD Fine Chemicals Ltd), directly used without drying
Procedure
Author Comments
- 4-Oxyquinazolines display anti-cancer (VEGFR2, EGFR and BRAF) and anti-HIV biological properties.
- The cyclization is proceeds through intramolecular electrophilic cyclization of Schiff base of thiophene-2-carbaldehyade and 2-aminobenzonitrile in methanol.
- Benzotriazole, an inexpensive, air & moisture stable compound and that exerts excellent ligand properties and widely being as a catalyst.
Data
1H NMR: (400 MHz, CDCl3) δ 8.08 (dd, J = 4.3, 3.3 Hz, 2H), 7.90 (d, J = 8.4 Hz, 1H), 7.79 – 7.73 (m, 1H), 7.47 (d, J = 4.9 Hz, 1H), 7.43 (d, J = 7.1 Hz, 1H), 7.17 – 7.13 (m, 1H), 4.22 (s, 3H).
13C NMR: (126 MHz, CDCl3) δ 166.87, 156.80, 151.66, 144.15, 133.64, 129.62, 128.97, 128.10, 127.52, 126.16, 123.53, 115.16, 54.21
IR (CHCl3) 3104, 2937, 1619, 1575, 1563, 1497, 1456, 1425, 1376 cm-1
ESI-MS: m/z 243 (M + 1)+; HRMS: m/z 243.0590 calcd for C13H10N2OS + H+ (243.0592).
Lead Reference
S. Battula, R. A. Vishwakarma, and Q. N. Ahmed. Synthesis Of 4-Methoxy-2-(Thiophene-2yl)-quinazoline by Cu-Benzotriazole catalyzed Electrophilic intramolecular cyclization of N-arylimines; RSC Adv. 2014, 4, 38375-38378.
Other References
(1) M. Joshi, R. Tiwari and A. K. Verma, Org. Lett., 2012, 14, 1106–1109
https://doi.org/10.1021/ol203491p
(2) J. Sun, D.-D. Li, J.-R. Li, F. Fang, Q.-R. Du, Y. Qian and H.-L. Zhu, Org. Biomol. Chem., 2013, 11, 7676-7686.
https://doi.org/10.1039/C3OB41136B
(3) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166
Supplementary Information
Keywords
4-Oxyquinazoline, Anti-cancer, Cu-Benzotriazole, heterocyclic compounds, substitution