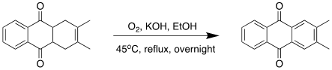
Oxidation of 1,4,4a,9a-tetrahydro-2,3-dimethyl-9,10-anthracenedione
SyntheticPage 937
Submitted: September 25, 2020, published: November 9, 2020
Authors
Autumn Peters (anpeters@millersville.edu)
Steven Kennedy (Steven.Kennedy@millersville.edu)
A contribution from
Chemicals
1,4,4a,9a-tetrahydro-2,3-dimethyl-9,10-anthracenedione [936]
Potassium Hydroxide (Carolina Biological Supply Company)
Ethanol (200 proof, Pharmco)
Diethyl Ether (BHT stabilized, Fisher Scientific)
Procedure
In a round-bottom flask with a reflux condenser, 1,4,4a,9a-tetrahydro-2,3-dimethyl-9,10-anthracenedione (0.24 g, 1 mmol) was dissolved in 3.6 mL of a 5% w/w ethanolic KOH solution. The solution was stirred and heated at 45 °C, overnight. After approx. 12 hours, the flask was removed from heat and allowed to cool to room temperature. The solid product was filtered using vacuum filtration and washed with water, ethanol, and diethyl ether. The product (2,3- dimethyl-9,10-anthracenedione) was allowed to air dry overnight yielding a light-green powder (0.21 g, 89%).
Author Comments
The reaction solution changes coloration from green initially to a distinct yellow following reaction completion.
This reaction has been performed from a 1 to 40 mmol scale where each reaction resulted in good yield and purity.
Residual water and ethanol peaks can be seen on the 1H NMR which can be resolved by further drying methods.
Data
1H NMR (400 MHz, CDCl3): δ 8.22 (t, 2H, J = 4.8 Hz), 8.10 (s, 2H), 7.78 (t, 2H, J = 4.4 Hz), 2.45 (s, 6H)
13C NMR (100 MHz, CDCl3): δ 183.34, 144.06, 142.37, 133.83, 133.66, 128.16, 127.06, 20.24
Rf = 0.39 (1:9 EtOAC/Hex)
M.p. 214.6 - 215.5°C
Lead Reference
Allen, C. F. H.; Bell, A. Organic Syntheses. 1942, 22, 37-8.
Supplementary Information
Keywords
alkenes, aromatics/arenes, ketones, oxidation