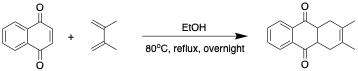
Diels-Alder reaction of 1,4-naphthoquinone with 2,3-dimethyl-1,3-butadiene
SyntheticPage 936
Submitted: September 25, 2020, published: October 5, 2020
Authors
Autumn Peters (anpeters@millersville.edu)
Steven Kennedy (Steven.Kennedy@millersville.edu)
A contribution from
Chemicals
1,4-Napthoquinone (>98%, TCI America)
2,3-dimethyl-1,3-butadiene (98%, stabilized with 100 ppm BHT, Alfa Aesar)
Ethanol (200 proof, Pharmco)
Procedure
To a stirred solution of 1,4-napthoquinone (6.33 g, 40 mmol) in ethanol (100 mL), 2,3-dimethyl-1,3-butadiene (4.95 mL, 44 mmol) was added via syringe. The solution was stirred, heated to 80 °C, and kept under reflux overnight. After approx. 12 hours at reflux, the reaction mixture was allowed to cool for 15 min before concentrating via rotary evaporation to yield the adduct (1,4,4a,9a-tetrahydro-2,3-dimethyl-9,10-anthracenedione) as a light gray, sand-colored solid (9.52 g, 99%).
Author Comments
Upon taking the reaction off of the heat, the solid crystalline product should begin to crash out. An ice bath may be used to aid in speed of this.
This reaction has been performed from a 2 to 40 mmol scale where each reaction resulted in good yield and purity.
Data
1H NMR (400 MHz, CDCl3): δ 8.22 (t, 2H, J = 4.8 Hz), 8.10 (s, 2H), 7.78 (t, 2H, J = 4.4 Hz), 2.45 (s, 6H)
13C NMR (100 MHz, CDCl3): δ 198.34, 134.18, 134.08, 126.81, 123.45, 47.34, 30.66, 18.87
Rf = 0.55 (1:3 EtOAC/Hex)
M.p. 146.2 - 147.1°C
Lead Reference
Allen, C. F. H.; Bell, A. Organic Syntheses. 1942, 22, 37-8.
Supplementary Information
Keywords
alkenes, cycloaddition, Diels-Alder, ketones