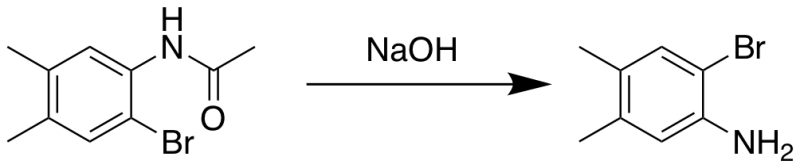
Amide hydrolysis of 2-bromo-4,5-dimethylacetanilide
SyntheticPage 933
Submitted: September 9, 2020, published: September 22, 2020
Authors
Steven Kennedy (Steven.Kennedy@millersville.edu)
Yongyu Ou (yoou@millersville.edu)
A contribution from
Chemicals
2-bromo-4,5-dimethylacetanilide [[932]]
NaOH (Spectrum Chemical)
Acetic Acid (Fisher Scientific)
Ethyl Acetate (99%, Alfa Aesar)
MgSO4 (EMD Millipore)
Procedure
2-bromo-4,5-dimethylacetanilide (0.24 g, 1 mmol) and 3 M NaOH (10 mL) were added into a 50 mL round bottom flask. The reaction was stirred at high speed and heated under reflux conditions overnight at 120°C, then cooled to room temperature. Acetic acid was added to neutralize the reaction to pH 7. The mixture was extracted with ethyl acetate (20 mL X 3) and water (20 mL). Then, MgSO4 was added to the combined ethyl acetate, filtered, then concentrated via rotary evaporation to give a crude product. Flash column chromatography (1:19 EtOAc/Hex) followed by vacuum concentration and drying provided a white solid product (0.14 g, 68 %)
Author Comments
Data
mp = 83.8°C - 85.0°C
Rf = 0.603 (1:9 EtOAc/Hex)
1 H NMR (400 MHz, CDCl3): δ 7.16 (s, 1H), 6.58 (s, 1H), 3.86 (s, 2H), 2.14 (s, 6H)
13 C NMR (100 MHz, CDCl3): δ 141.64, 136.80, 132.85, 127.94, 117.24, 106.13, 19.42, 18.48
Lead Reference
Claffey, Michelle Marie; Goldstein, Steven Wayne; et al. Aryl-substituted imidazo[1,2-a]pyridine derivatives as C3a receptor antagonists, their preparation, pharmaceutical compositions, and use in therapy. Patent WO 2007034282, A2, March 29, 2007.
Supplementary Information
Keywords
amide hydrolysis, amides, amines, aromatics/arenes, nucleophilic acyl substitution, substitution