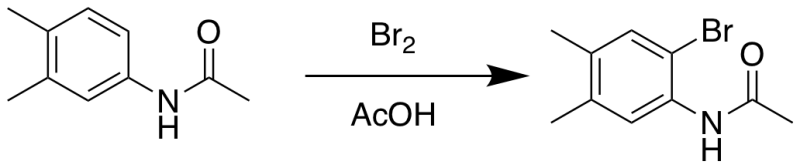
Bromination of 3,4-dimethylacetanilide
SyntheticPage 932
Submitted: September 9, 2020, published: September 22, 2020
Authors
Steven Kennedy (Steven.Kennedy@millersville.edu)
Yongyu Ou
A contribution from
Chemicals
3,4-dimethylacetanilide [[931]]
Acetic acid (Fisher Scientific)
Bromine
Ethanol (Pharmco)
Procedure
In a 250 mL round bottom flask, 3,4-dimethylacetanilide (1.47 g, 9.0 mmol) was dissolved in acetic acid (12.8 mL), then bromine (0.89 mL, 17.3 mmol) in acetic acid (3.18 mL) was added slowly over 10 minutes. After the reaction mixture was stirred overnight at room temperature, water (130 mL) was added, and a yellow precipitate formed. The precipitate was separated from the mixture solution by vacuum filtration using a fritted filter funnel. The precipitate was purified via recrystallization from ethanol and then triturated with ice-cold ethanol (less than 2 mL), which was stirred in an ice bath, then collected via filtration to obtain a pale-yellow product (1.2 g, 54 %), which was dried under vacuum.
Author Comments
The product had small amount of impurity as seen in the 1 H NMR spectra.
Data
mp = 155.9°C - 160.9°C
Rf = 0.37 (1:2 EtOAc/Hex)
1 H NMR (400 MHz, CDCl3): δ 8.06 (s, 1H), 7.44 (s, 1H), 7.28 (m, 1H), 2.22 (s, 3H), 2.21 (s, 3H), 2.20 (s, 3H)
13 C NMR (100 MHz, CDCl3): δ 168.02, 137.00, 134.09, 133.15, 132.48, 123.13, 110.06, 24.77, 19.67, 19.03
Lead Reference
Claffey, Michelle Marie; Goldstein, Steven Wayne; et al.. Aryl-substituted imidazo[1,2-a]pyridine derivatives as C3a receptor antagonists, their preparation, pharmaceutical compositions, and use in therapy. Patent WO 2007034282, A2, March 29, 2007.
Supplementary Information
Keywords
amides, amines, aromatics/arenes, bromination