Deprotection of [[923|ethyl (2S)‐2‐[(2S)‐2‐[2‐(naphthalen‐2‐yloxy)acetamido]‐3‐phenylpropanamido]‐3‐phenylpropanoate]]
SyntheticPage 924
Submitted: April 27, 2020, published: May 4, 2020
Authors
Bart Dietrich (bart.dietrich@yahoo.co.uk)
A contribution from
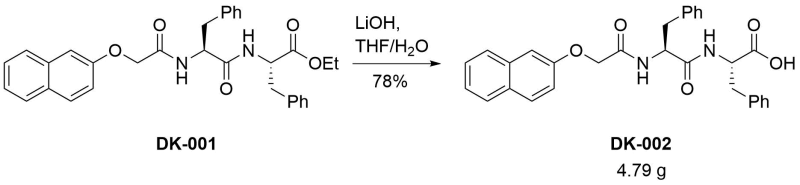
Chemicals
Lithium hydroxide anhydrous (Alfa)
Tetrahydrofuran HPLC grade (Fisher)
Water (Scottish quality tap water)
Procedure
To a solution of the starting material (6.50 g, 12.4 mmol) in tetrahydrofuran (100 mL) was added a solution of lithium hydroxide (4 eq, 1.19 g) in water (100 mL) and the cloudy mixture was stirred overnight. After this time, the clear solution was poured into 1M hydrochloric acid (ca. 600 mL) and stirred for 90 minutes. The resulting precipitate was filtered off, washed in the filter with water, then dried by azeotropic distillation with acetonitrile. The title compound was thus obtained as a white solid (4.79 g, 78 %) in sufficient purity (< 0.4 % acetonitrile by NMR).
Author Comments
- The reaction conditions may seem rather dilute but they work. At high concentrations of lithium hydroxide amide bond cleavage can begin to be a problem.
- The reaction does not usually require overnight stirring. The reaction is mostly complete after it becomes clear. At this stage, a small aliquot can be transferred to a vial and 1M HCl added. If a powdery (not sticky) precipitate is seen, the reaction can be assumed to have reached completion. If the NMR of the product shows the presence of starting material, the material can simply be returned to THF/LiOH/water and stirred for longer.
- Hydrated forms of lithium hydroxide may be used as well. The weight needs to be recalculated accordingly.
Data
dH (400 MHz, DMSO-d6) 12.81 (1H, br s, COOH), 8.44 (1H, d, J 7.84, NaH), 8.12 (1H, d, J 8.56, NbH), 7.84-7.82 (2H, m, HAr), 7.72 (1H, d, J 8.12, HAr), 7.48-7.44 (1H, m, HAr), 7.38-7.34 (1H, m, HAr), 7.26-7.13 (12H, m, HAr), 4.64 (1H, dt, J 9.08, 4.16, CbH*), 4.53 (2H, s, OCH2), 4.50-4.44 (1H, m, CaH*), 3.07 (1H, dd, J 14.02, 5.34, PhCH’H”CaH*), 3.02 (1H, dd, J 14.16, 4.40, PhCH’H”CbH*), 2.92 (1H, dd, J 13.92, 8.76, PhCH’H”CaH*), 2.85 (1H, dd, J 13.82, 9.62, PhCH’H”CbH*).
dC (100 MHz, DMSO-d6) 172.76, 170.88, and 167.24 (C=O), 155.51, 137.54, 137.38, 134.05, 129.38, 129.28, 129.15, 128.77, 128.21, 128.00, 127.53, 126.82, 126.48, 126.45, 126.28, 123.88, 118.48, and 107.34 (CAr), 66.70 (OCH2), 53.53 (CaH*), 53.26 (CbH*), 37.45 (PhCH2CaH*), 36.71 (PhCH2CbH*).
HRMS (ESI) m/z: [M+Na]+ calcd for C30H28N2NaO5 519.1890; found 519.1907.
Keywords
2NapFF, 2-NapFF, amino acids, ester deprotection, gel, gelator, gels, NapFF, peptides