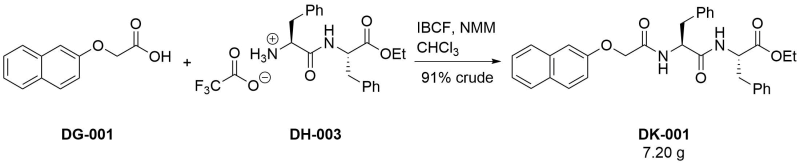
Coupling of [[922|ethyl (2S)‐2‐[(2S)‐2‐amino‐3‐phenylpropanamido]‐3‐phenylpropanoate trifluoroacetate salt]] with 2-naphthoxyacetic acid
SyntheticPage 923
Submitted: April 27, 2020, published: May 4, 2020
Authors
Bart Dietrich (bart.dietrich@glasgow.ac.uk)
A contribution from
Chemicals
Isobutyl chloroformate (Fluorochem)
N-Methylmorpholine (TCI)
Chloroform (HPLC grade)
Procedure
To a suspension of 2-naphthoxyacetic acid (3.03 g, 15.0 mmol) in chloroform (50 mL) were added iso-butyl chloroformate (1 eq, 1.95 mL) and N-methylmorpholine (1 eq, 1.65 mL) and the mixture was stirred for one hour. A solution of [[922|ethyl (2S)‐2‐[(2S)‐2‐amino‐3‐phenylpropanamido]‐3‐phenylpropanoate trifluoroacetate salt]] (1 eq, 6.82 g) and N-methylmorpholine (1 eq, 1.65 mL) in chloroform (50 mL) was then added and the reaction was stirred for 3 days at room temperature. After this time, it was diluted with chloroform and washed in turn with 1M hydrochloric acid, saturated aqueous sodium carbonate solution, water, and brine. After drying over magnesium sulfate and evaporation in vacuo, the crude title compound was obtained as a light-brown solid (7.20 g, 91 %). This was used in the next step without further purification. A small amount was purified via column chromatography (eluting with 1:99 ethyl acetate/dichloromethane) to yield a sample suitable for characterisation.
Author Comments
- The three days reaction time is not necessary; it happened to be a weekend. The reaction is usually stirred overnight but is generally complete after an hour or two (TLC).
- The formation of a pink-coloured impurity can be reduced somewhat by carrying out the reaction at ice/water temperature, although the gains are minimal.
- The crude product can be used in the next step without purification.
Data
dH (400 MHz, DMSO-d6) 8.61 (1H, d, J 7.48, NH), 8.16 (1H, d, J 8.56, NH), 7.85-7.82 (2H, m, HAr), 7.73 (1H, d, J 8.08, HAr), 7.46 (1H, ddd, J 8.09, 6.91, 1.15, HAr), 7.36 (1H, ddd, J 8.04, 6.92, 1.12, HAr), 7.28-7.12 (12H, m, HAr), 4.66 (1H, td, J 9.04, 4.20, CH*), 4.54 (2H, s, OCH2), 4.51-4.46 (1H, m, CH*), 4.04 (2H, q, J 7.11, CH2CH3), 3.05-2.93 (3H, m, PhCaH2 and PhCbHaHb), 2.85 (1H, dd, J 13.86, 9.70, PhCbHaHb), 1.09 (3H, t, J 7.10, CH2CH3).
dC (100 MHz, DMSO-d6) 171.19, 170.95, and 167.23 (C=O), 155.48, 137.45, 136.93, 134.01, 129.32, 129.17, 129.07, 128.73, 128.20, 127.96, 127.47, 126.76, 126.53, 126.38, 126.26, 123.82, 118.43, and 107.32 (CAr), 66.68 (OCH2), 60.51 (CH2CH3), 53.69 (CH*), 53.16 (CH*), 37.45 (PhCbH2), 36.68 (PhCaH2), 13.89 (CH2CH3).
HRMS (ESI) m/z: [M+Na]+ calcd for C32H32N2NaO5 547.2203; found 547.2197.
Keywords
amide coupling, amino acids, peptides