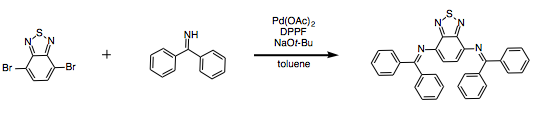
Buchwald-Hartwig coupling of benzophenone imine with 4,7-dibromobenzo[c]-1,2,5-thiadiazole
SyntheticPage 909
DOI:
Submitted: August 26, 2019, published: September 5, 2019
Authors
Kathryn Allen (kathryn.allen@millersville.edu)
Steven Knauss (sjknaus1@millersville.edu)
A contribution from
Chemicals
4,7-Dibromobenzo[c]-1,2,5-thiadiazole, 95%, Aldrich
1,1′-Ferrocenediyl-bis(diphenylphosphine) (DPPF), 97%, Aldrich
Palladium (II) acetate, reagent grade, 98%, Aldrich
Sodium tert-butoxide, 97%, Aldrich
Benzophenone imine, 95%, Aldrich
Procedure
An oven-dried three-necked round bottom flask and stir bar was equipped with three septa and purged with dry nitrogen gas for 30 minutes. Anhydrous toluene (5.6 mL, 0.18 M), 4,7-dibromobenzo[c]-1,2,5-thiadiazole (293.9 mg, 1.00 mmol, 1 eq.), DPPF (27.7 mg, 0.05 mmol), palladium (II) acetate (16.8 mg, 0.075 mmol), and sodium tert-butoxide (211.4 mg, 2.20 mmol, 2.2 eq.) were added to the flask. Benzophenone imine (0.34 mL, 2.00 mmol, 2 eq.) was added dropwise by syringe under nitrogen. The solution was heated to 90°C for 24 hours. On workup, the solution was diluted with water, and washed with ethyl acetate. The water layer was washed with dichloromethane. The organic layers were combined, dried over magnesium sulfate and filtered. The solvent was removed under reduced pressure to yield a thick, dark red oil. The oil was taken up in hot hexanes and cooled to room temperature and filtered to yield a brick-red powder (326.8 mg, 0.66 mmol, 66% yield).
Author Comments
Data
1H NMR (400 MHz, CDCl3) δ 7.820 (d, J = 8 Hz, 4H), 7.491 (m, 2H), 7.411 (m, 4H), 7.209 (m, 2H), 7.129 (m, 4H), 7.057 (d, J = 8 Hz, 4H), 6.456 (s, 2H).
13C NMR (400 MHz, CDCl3) δ 171.090, 149.635, 139.313, 138.911, 136.346, 131.132, 129.690, 128.680, 128.187, 127.792, 117.220.
Infrared Spectra
1607 cm-1 (imine C=N), 1596 cm-1, 1577 cm-1 (BTD C=N)
High-Resolution Mass Spectrometry
Calculated: 494.1565 m/z
Found (M + 1)+: 495.1635 m/z
Lead Reference
Keywords
heterocyclic compounds, imine, transition metal catalysed