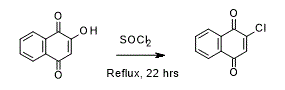
Chlorination of 2-Hydroxy-1,4-napthoquinone
SyntheticPage 886
DOI:
Submitted: March 29, 2019, published: April 17, 2019
Authors
Cameron Zinkel
Charnete Casimero
James Davis (James.Davis@ulster.ac.uk)
Robert Smith (rbsmith@uclan.ac.uk)
A contribution from
Chemicals
2-Hydroxy-1,4-naphthoquinone (Sigma Aldrich)
Thionyl chloride (Sigma Aldrich)
Diethyl ether (Fisher Scientific)
Procedure
To a round-bottomed flask was charged 2-hydroxy-1,4-napthoquinone (3.00 g, 17.2 mmol) and thionyl chloride (30 mL, 413.4 mmol), the reaction mixture was heated to reflux with constant stirring for 22 hours*. Upon cooling the reaction mixture was evaporated to dryness** and the dark yellow solid produced was washed with diethyl ether to yield 2-chloro-1,4-napthoquinone (2.84 g, 86%) as an orange solid.
Author Comments
*A round-bottomed flask fitted with a reflux condenser was used which fitted snugly into a metal heating block. A thermocouple was used to control the temperature and the reaction was open to the air (no inert gasses were required). Stirring was accomplished using a stirring bar, which was controlled from the hot plate. The whole reaction was accomplished within the fume cupboard without the need of dry/inert conditions. Also, the reaction mixture went into solution upon being heated and remained in solution upon cooling after the 22 hours. The reaction was kept in the fume cupboard at all times during the addition of the thionyl chloride, as well as the reflux and subsequent cooling stage.
** Removal of thionyl chloride was accomplished under reduced pressure within a closed (sash) fume cupboard. The removal of thionyl chloride was accomplished on a rotor evaporator with full safety clips to secure the flask, with a temperature-controlled water-bath. The reaction was placed in the cold water bath and during the evaporation the bath gradually was increased to 50°, with the first collection trap being cooled in ice water to isolate the thionyl chloride. The thionyl chloride itself was not reused after the reaction and disposal was in accordance with institutional disposal procedures.
Data
1H NMR (300 MHz, Chloroform-d) δ 8.25 – 8.16 (m, 1H), 8.16 – 8.07 (m, 1H), 7.86 – 7.76 (m, 2H), 7.25 (s, 1H). 13C NMR (75 MHz, CDCl3) δ 182.73, 178.01, 146.35, 135.95, 134.56, 134.19, 131.75, 131.29, 127.56, 126.79.
Lead Reference
S. Neufeind, N. Hülsken, J.-M. Neudörfl, N. Schlörer, H.-G. Schmalz Total synthesis of cyclo-mumbaistatin analogues through anionic homo-fries rearrangement, Chem. Eur J., 17 (2011), pp. 2633-2641
Other References
Supplementary Information
Keywords
alcohols, aromatics/arenes, chlorination