Reduction of 1,8-Dihydroxyanthraquinone
SyntheticPage 875
DOI:
Submitted: January 7, 2019, published: January 9, 2019
Authors
Clare Lawrence (CLLawrence@uclan.ac.uk)
Megan Critchley (MCritchley@uclan.ac.uk)
Robert Smith (rbsmith@uclan.ac.uk)
A contribution from
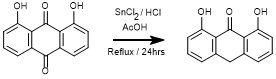
Chemicals
1,4-Dihydroxyanthraquinone (Sigma Aldrich).
Glacial acetic acid (Fisher Scientific).
Anhydrous tin (II) chloride (Sigma Aldrich).
Concentrated hydrochloric acid (Fisher Scientific).
Procedure
To a round-bottomed flask* charged with 1,8-dihydroxyanthraquinone (0.24 g, 1 mmol) and glacial acetic acid (20 mL) was slowly added a warm** solution of anhydrous tin (II) chloride (2.14, 11.29 mmol) in conc. hydrochloric acid (11 mL). The reaction mixture was heated at reflux for 24 hours with constant stirring. The reaction mixture was allowed to cool and slowly poured over ice water to yield a yellow solid. The solid was isolated by vacuum filtration on a Buchner funnel to yield 1,8-dihydroxyanthrone (0.20g, 88%) as a yellow solid.
Author Comments
*A round-bottomed flask fitted with a reflux condenser was used which fitted snugly into a metal heating block. A thermocouple was used to control the temperature and the reaction was open to the air (no inert gasses were required). Stirring was accomplished using a stirring bar, which was controlled from the hot plate. The whole reaction was accomplished in the open air without the need of dry/inert conditions.
** The solution was at 40°C.
Data
1H NMR (300 MHz, DMSO-d6) δ 12.09 (s, 2H), 7.61 (t, J = 7.9 Hz, 2H), 7.03 (dd, J = 7.6, 1.3 Hz, 2H), 6.90 (d, J = 8.3 Hz, 2H), 4.47 (s, 2H). 13C NMR (75 MHz, DMSO) δ 194.05, 162.29, 143.31, 137.17, 119.65, 115.77, 115.32, 32.73. GC-MS EI [M]: 226.73
Lead Reference
Jiro Motoyoshiya, Yusuke Masue, Yoshinori Nishi and Hiromu Aoyama, Synthesis of Hypericin via Emodin Anthrone Derived from aTwo-fold Diels-Alder Reaction of 1,4-Benzoquinone, Natural Product Communications, 2007, 2(1), 67 – 70.
Keywords
aromatics/arenes, heterocyclic compounds, ketones, reduction