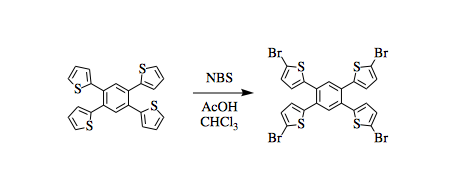
Bromination of tetrathiophenylbenzene
SyntheticPage 872
DOI:
Submitted: December 5, 2018, published: January 1, 2019
Authors
Kathryn Allen (kathryn.allen@millersville.edu)
Liam Schroeder (lfschroe@millersville.edu)
A contribution from
Chemicals
1,2,4,5-tetra(thiophen-2-yl)benzene, see ChemSpider Synthetic Pages 871
N-Bromosuccinimide, ReagentPlus, 99% Aldrich
Chloroform, Certified ACS Approx. 0.75% ethanol as preservative, Fisher Chemical
Acetic Acid, Glacial, Fisher Chemical
Procedure
A three-necked round-bottomed flask was equipped with a stir bar and condenser. Chloroform (25 mL) and acetic acid (25 mL) were added to the reaction flask, then 1,2,4,5-tetra(thiophen-2-yl)benzene (0.830g, 2 mmol, 1 eq.) and N-bromosuccinimide (1.817g, 10 mmol, 5 eq.) were added. The reaction was heated at 75°C for 17 hours in air. Reaction completion was monitored by 1H NMR of an aliquot every two hours. Upon completion of the reaction, the flask was cooled to room temperature and more chloroform was added until all the solid had dissolved. The organic layer was washed with saturated sodium bisulfite solution (2 x 200 mL) and saturated potassium carbonate solution (2 x 200 mL). The organic layer was dried over sodium sulfate, filtered, and concentrated under reduced pressure on a rotary evaporator. The product was recrystallized from 25% ethyl acetate in hexanes to yield a green-brown powder (1.079 g, 1.49 mmol, 75% yield).
Author Comments
Acetic acid greatly accelerates this reaction. Brominating in chloroform yielded little to no product. The reaction was generally done in five hours, but did not overbrominate if left over the weekend.
Please note: NBS must be recrystallized from water before being used in order to receive the best results.
NMR analysis: an aliquot was removed, dried on a hot air gun, and NMR was taken in CDCl3, specifically to monitor for the loss of the thienyl proton in the 5 position.
The authors thank Dr. Daniel Ralston for DART MS collection.
Please note: NBS must be recrystallized from water before being used in order to receive the best results.
NMR analysis: an aliquot was removed, dried on a hot air gun, and NMR was taken in CDCl3, specifically to monitor for the loss of the thienyl proton in the 5 position.
The authors thank Dr. Daniel Ralston for DART MS collection.
Data
1H NMR (400 MHz, CDCl3): δ 7.501 (s, 2H), 6.964 (d, 4H, J = 3.6 Hz), 6.744 (d, 4H, J = 3.6 Hz)
13C NMR (400MHz, CDCl3): δ 141.607, 133.408, 133.264, 127.426, 127.047, 126.361.
Mass Spec: Calculated: 722.1780 g/mol; observed: 722.6476 g/mol.
Keywords
aromatics/arenes, bromination, heterocyclic compounds