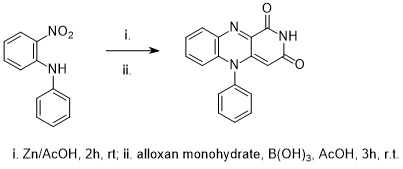
Reduction of 2-nitrophenylamine followed by cyclocondensation with alloxan monohydrate
SyntheticPage 844
DOI:
Submitted: August 7, 2018, published: September 4, 2018
Authors
Clare Lawrence (CLLawrence@uclan.ac.uk)
Matthew Dickinson (MDickinson1@uclan.ac.uk)
Megan Critchley (MCritchley@uclan.ac.uk)
Robert Smith (rbsmith@uclan.ac.uk)
A contribution from
Chemicals
2-Nitrodiphenylamine 98%, (Sigma Aldrich)
Zinc dust (Sigma Aldrich)
Alloxan monohydrate (Alfa Aesar)
Procedure
To a round bottom flask was added 2-nitrophenylamine (2.14g, 10 mmol) and glacial acetic acid (30mL). The solution was lowered into a slush bath and with constant stirring, zinc dust was added gradually (13.0 g, 200 mmol).* Upon the total addition of zinc dust, the reaction was removed from the slush bath and stirred at room temperature for two hours. The solution was poured over celite at the pump and the cake was washed with glacial acetic acid (10mL).
The freshly filtered solution was added to a mixture of alloxan monohydrate (1.59g, 10mmol) and boric acid (0.92g, 15mmol). The mixture was stirred for three hours, to produce a yellow crude precipitate which was isolated at the pump and washed with diethyl ether to yield 10-phenylisoalloxazine (2.74 g, 94%) as a bright yellow solid which was dried in vacuo.
Author Comments
*Note that a rapid temperature rise is seen upon the addition of the zinc dust. The solution should be monitored in the slush bath, as acetic acid freezes below 16 ºC and as such the solution may need to be removed at points in order to maintain a solution. Should an exothermic reaction be noted whilst the solution is outside the slush bath, then the reaction mixture should be lowered back in the slush bath. Note the zinc dust was added via a micro spatula, one portion at a time with the total addition taking around 10 min (dependant on controlling temperature). No issues were noted on stirring, but a rapid stir is encouraged.
The compound can be recrystallized from dissolving in boiling acetic acid, with the 10-phenylisoalloxazine crystals dropping out of solution on cooling.
Signals on NMR which are not recorded: 1H = 1.81ppm, 13C – 22.88 & 177.12 are deemed to be acetic acid.
Data
1H NMR (400 MHz, DMSO-d6) δ 11.44 (s, 1H), 8.18 (d, J = 8.1 Hz, 1H), 7.81 – 7.55 (m, 6H), 7.44 (d, J = 7.6 Hz, 2H), 6.75 (d, J = 8.5 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 160.00, 156.01, 152.19, 139.95, 136.55, 135.24, 135.17, 134.50, 131.83, 130.79, 130.27, 128.29, 126.47, 117.22. LCMS m/z: 290 [M+], 291 found. IR (cm-1): 3153.63, 3095.11, 3033.15, 2826.57, 2552.63, 2365.45, 2191.48, 1958.40, 1660.97, 1581.04, 1506.84, 1490.91, 1268.62, 1195.79, 877.15
Lead Reference
Mallesham Bejugam, Sven Sewitz, Pravin S. Shirude, Raphaël Rodriguez, Ramla Shahid, and Shankar Balasubramanian. Trisubstituted Isoalloxazines as a New Class of G-Quadruplex Binding Ligands: Small Molecule Regulation of c-kit Oncogene Expression. J Am Chem Soc. 2007 Oct 31; 129(43): 12926–12927.
Supplementary Information
Keywords
amines, aromatics/arenes, cyclization, cyclocondensation, heterocyclic compounds, ketones, reduction