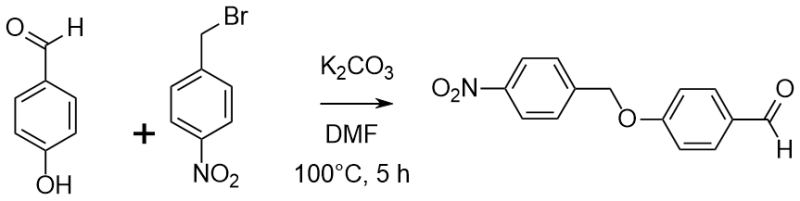
Etherification of 4-Hydroxybenzaldehyde with a benzyl bromide
SyntheticPage 836
DOI:
Submitted: June 8, 2018, published: June 9, 2018
Authors
Megan Critchley (MCritchley2@uclan.ac.uk)
Robert Smith (rbsmith@uclan.ac.uk)
A contribution from
Chemicals
4-Hydroxybenzaldehyde (Sigma Aldrich)
4-nitrobenzyl bromide (Sigma Aldrich)
Potassium carbonate (Sigma Aldrich)
DMF (Sigma Aldrich)
Procedure
A solution of 4-hydroxybenzaldhyde (2.0 g, 16.4 mmol), 4-nitrobenzyl bromide (3.54 g, 16.4 mmol), potassium carbonate (3.40 g, 24.6 mmol) in dry DMF (20 mL) was heated at 100℃ for 3 h with constant stirring. The reaction mixture was allowed to cool, added to ice water and the resulting precipitate was isolated at the pump to give a yellow solid. The solid was washed three times with water (10 mL each time) to yield 4-(4-nitrobenzyloxy)benzaldehyde (3.11 g, 74%) as a light yellow solid. [How was it dried?]
Author Comments
The title compound can be recrystallized either using 1:1 mixture of hexane : toluene [please give brief protocol] or purified by column chromatography on silica gel using n-hexane/EtOAc mixture as a solvent system. The authors found the above synthesis to not require purification, as the compound produced was sufficiently pure to act as an intermediate for the next stage of the synthesis.
Data
1H NMR (300 MHz, DMSO-d6) δ 9.88 (s, 1H), 8.27 (d, J = 8.7 Hz, 2H), 7.89 (d, J = 8.7 Hz, 2H), 7.74 (d, J = 8.7 Hz, 2H), 7.23 (d, J = 8.7 Hz, 2H), 5.41 (s, 2H). 13C NMR (75 MHz, DMSO) δ 191.83, 163.25, 147.56, 144.66, 132.32, 130.50, 128.85, 124.15, 115.78, 68.83. MS m/z: 257.22 [M+]. IR (cm-1): 3060.96, 2926.67, 1686.57, 1595.79, 1160.45.
Lead Reference
Kafle, B., Aher, NG., Khadka, D., Park, H., Cho, H. Isoxazol-5(4H)one Derivatives as PTP1B Inhibitors Showing an Anti-Obesity Effect, Chem Asian J., 2011, 6(8):2073-9
Supplementary Information
Keywords
aldehydes, alkyl/alkenyl/aryl halides, aromatics/arenes, substitution