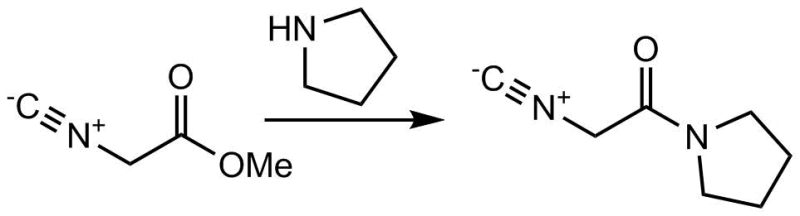
Aminolysis of methyl isocyanoacetate with pyrrolidine
SyntheticPage 833
DOI:
Submitted: February 9, 2018, published: February 12, 2018
Authors
Abhishek Purohit (apurohit1@gulls.salisbury.edu)
Stephen Habay (sahabay@salisbury.edu)
Chemicals
Methyl isocyanoacetate (95 %, Acros)
Pyrrolidine (99 %, Sigma-Aldrich)
Pyrrolidine (99 %, Sigma-Aldrich)
Procedure
To a flask of magnetically stirred pyrrolidine (4.19 mL, 51.2 mmol) was added dropwise methyl isocyanoacetate (4.9 mL, 51.2 mmol). The reaction was stirred at room temperature for 20 min or until the solution solidified. The solid residue was dissolved in ethyl acetate (100 mL) and vacuum filtered through a 1 inch deep plug of silica gel. The silica plug was washed with two additional portions of ethyl acetate (50 mL). The combined filtrate was concentrated by rotary evaporation, leaving a tan solid product (6.58 g, 93 %), which was dried under vacuum .
Author Comments
On larger scales, the reaction flask is immersed in an ambient temperature water bath during the addition of methyl isocyanoacetate to prevent a large temperature change.
The color of the product can vary from light yellow to orange-brown.
The color of the product can vary from light yellow to orange-brown.
Data
mp = 69-72 °C
IR (ATR): 2978, 2162, 1653, 1448, 1415, 1329 cm-1
1H-NMR (400 MHz, CDCl3): δ ppm 4.22 (s, 2 H) 3.50 (t, J = 6.9 Hz, 2 H) 3.38 (t, J = 6.8 Hz, 2 H) 1.94 - 2.05 (m, 2 H) 1.83 - 1.93 (m, 2 H)
13C-NMR (100 MHz, CDCl3): δ ppm 160.52, 160.23, 46.49, 46.09, 44.91, 26.01, 23.93
IR (ATR): 2978, 2162, 1653, 1448, 1415, 1329 cm-1
1H-NMR (400 MHz, CDCl3): δ ppm 4.22 (s, 2 H) 3.50 (t, J = 6.9 Hz, 2 H) 3.38 (t, J = 6.8 Hz, 2 H) 1.94 - 2.05 (m, 2 H) 1.83 - 1.93 (m, 2 H)
13C-NMR (100 MHz, CDCl3): δ ppm 160.52, 160.23, 46.49, 46.09, 44.91, 26.01, 23.93
Lead Reference
Dömling, A; Beck, B.; Fuchs, T.; Yazbak, A. J. Comb. Chem., 2006, 8, 872–880.
Other References
Mossetti, R.; Caprioglio, D.; Colombano, G.; Tron, G.C.; Pirali, T. Org. Biomol. Chem., 2011, 9, 1627-1631.
Keywords
amides, esters, isocyanide, isonitrile, nucleophilic, substitution