Reductive Amination of Tryptophan
SyntheticPage 830
DOI:
Submitted: September 22, 2017, published: October 5, 2017
Authors
Andrew Schneerer (schne586@umn.edu)
Nathaniel Olson (olso4006@umn.edu)
Wayland Noland (nolan001@umn.edu)
A contribution from
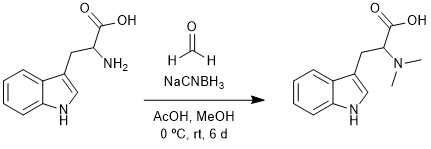
Chemicals
Methanol (ACS grade, 99.8%; Aldrich)
Sodium Cyanoborohydride
Acetic Acid (ACS grade, 99.7%; Mallinckrodt)
Formaldehyde
Procedure
Author Comments
Data
Rf = 0.10 (Al2O3, 17:2:1 CHCl3:MeOH:AcOH
M.p. 230 ºC dec.
1H NMR (500 MHz, DMSO): δ 10.887 (s, br., 1H), 7.551 (d, J = 8.0, 1H), 7.335 (d, J = 8.3), 7.178 (d, J = 2.2, 1H), 7.059 (t, J = 7.5), 6.974 (t, J = 7.5, 1H), 3.504 (dd, J = 8.0, 6.1, 1H), 3.189 (dd, J = 14.7, 8.3, 1H), 2.997 (dd, J = 14.8, 6.0, 1H), 2.440 (s, 6H)
13C NMR (126 MHz, DMSO) δ 170.94, 136.07, 127.17, 123.46, 120.83, 118.26, 118.23, 111.35, 110.37, 68.53, 41.28, 24.32
IR (KBr, cm-1) 3244, 3048, 2959, 2919, 1623, 1484, 1458, 1439, 1352, 1288, 1239, 1172, 1147, 1032, 1013, 769, 756, 738 cm-1
MS (CI, m/z) [M-CO2]+ calculated for C12H17N2+ 189.1386, found 189.1386.
Anal. Calcd for C13H16N2O2: C, 67.22, H, 6.94, N, 12.06. Found: C, 66.95, H, 6.91, N, 12.01
Lead Reference
Supplementary Information
Keywords
aldehydes, amines, heterocyclic compounds, indole, reductive amination, tryptophan