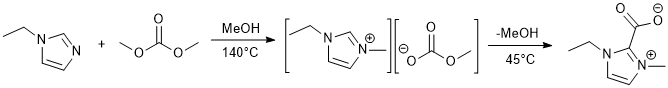
Alkylation and carboxylation of 1-ethylimidazole
SyntheticPage 828
DOI:
Submitted: June 19, 2017, published: June 21, 2017
Authors
Marianne Kjellberg (marianne.kjellberg@orange.fr)
Chemicals
dimethylcarbonate
methanol
acetonitrile
Procedure
In a 1000 mL Parr reaction vessel, 1-ethylimidazole (43.15 g, 449 mmol) (Note 1) was added to dimethylcarbonate (95 g, 1055 mmol) with 290 mL of methanol. The reactor was sealed and heated at 140°C for 25 hours in the circular heater resulting in 298.35 g of a pale yellow liquid. The conversion of ethylimidazole was complete (Note 2).
The 1-ethyl-3-methylimidazolium methylcarbonate (100.46 g, 27.9 g of [C2C1im][MeCO3], 150 mmol, in methanol) was dried over 3Å molecular sieves for 72 hours (Note 3). The methanol was then removed by stirring the solution in a dry 250 mL three-neck flask under vacuum at 45°C in a glycerol bath. The viscous, dark brown remaining liquid gave 22.55 g of a mustard yellow solid upon cooling at room temperature under vacuum. The solid was then recrystallized (Note 4) three times under nitrogen using dry acetonitrile (0.5 mL of solvent for 1 g of solid) (Note 5). The first time a brief quench in a dry ice and acetone bath was needed to quicken the crystallization, but the subsequent times cooling at room temperature was sufficient. After each recrystallization the solvent was removed via canula under nitrogen and the remaining solid was washed with 3*10 mL of dry acetonitrile under nitrogen. After 24 hours under vacuum at 45°C, the solid (off-white crystals) was weighed inside a glovebox (6.42 g). Total yield from 1-ethylimidazole : 28%.
Author Comments
- 1-ethylimidazole was freshly distilled from KOH
- methanol was drawn from a new bottle - Karl-Fischer titration showed less than 100 ppm water.
- dimethylcarbonate was dried over 3Å molecular sieves prior to use
2: according to NMR of the methanolic liquor. Shifts for [C2C1im][MeCO3] are as following (abstracting from methanol and excess dimethylcarbonate in the liquor) :
1H NMR (400 MHz, DMSO-d6) δ 9.29 (s, 1H), 7.78 (d, 1H, J = 2.0 Hz), 7.69 (d, 1H, J = 2.0 Hz), 4.21 (q, 2H, J = 7.3 Hz), 3.86 (s, 3H), 3.28 (m, 3H), 1.42 (t, J = 7.3 Hz, 3H).
13C NMR (101 MHz, DMSO-d6) δ 157.00, 137.77, 124.73, 123.13, 52.48, 45.34, 36.66, 16.15.
3: Particular care should be taken towards the ingress of water in the second step of the process : all glassware must be oven-dried prior to use, and the content of the flask should never be exposed to air - the flask must always be opened under a stream of nitrogen. This is because the solid is very hygroscopic and water would convert the zwitterion into the corresponding hydrogen carbonate salt.
4. Recrystallization: use the right amount of acetonitrile. Too much solvent would decrease the yield dramatically, while too little solvent would not be enough to solubilise most of the impurity. The right amount should be between 0.25 and 1 mL of solvent per gram of solid. After the last recystallisation and washings, off-white crystals should remain - not compact yellowish rock.
5. The acetonitrile was dried over alumina and stored over 3Å molecular sieves prior to use.
Data
1H NMR (400 MHz, DMSO-d6) δ 7.65 (d, J = 2.0 Hz, 1H), 7.57 (d, J = 2.0 Hz, 1H), 4.48 (q, J = 7.2 Hz, 2H), 3.96 (s, 3H), 1.35 (t, J = 7.2 Hz, 3H).
13C NMR (101 MHz, DMSO-d6) δ 154.94, 142.78, 123.23, 121.30, 44.94, 41.08, 40.87, 40.66, 40.45, 40.24, 40.03, 39.83, 37.38, 16.87.
Lead Reference
Other References
Keywords
addition, aromatics/arenes, carbonates, carboxylation, heterocyclic compounds, NHC-CO2 adducts, thermal, zwitterion