Conjuage addition of a cuprate to an enamide
SyntheticPage 82
DOI:
Submitted: August 17, 2001, published: August 17, 2001
Authors
Mala Mistry (m.mistry@sussex.ac.uk)
A contribution from
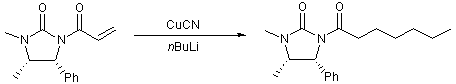
Chemicals
Copper cyanide (Aldrich)
nButyl lithium (1.6M in Hexane, Acros)
Tetrahydrofuran (distilled from sodium benzophenone)
nButyl lithium (1.6M in Hexane, Acros)
Tetrahydrofuran (distilled from sodium benzophenone)
Procedure
A solution of copper cyanide (0.1 g, 1.11 mmol, 1 eq.) in THF (3 mL) was cooled to -78oC, to which was added nBuLi (1.4 mL, 2.23 mmol, 2 eq.) dropwise. The hetrogenous mixture was warmed to 0oC and stirred for about 10 minutes. The now homogenous mixture was recooled to -78oC and a solution of the enamide (0.22 g, 0.925 mmol, 1 eq) in THF (3 mL) was added by cannula slowly. The reaction vessel was removed from the ice bath and allowed to warm to room temperature and stirred for about one hour (progress was checked by TLC 5:1 PE:EtOAc to ensure completion of the reaction).
The reaction mixture was quenched by the addition of saturated ammonium chloride solution (5 mL) and diluted with EtOAc (5 mL). The organic extracts were dried, filtered and the solvent removed in vacuo. Purification by flash column chromatography is necessary to remove any copper impurities (10:1-0:1 PE:EtOAc) to afford a colourless oil in average yields of 45 % (115 mg).
Author Comments
In this particular conjugate addition reaction it is OK for the solution to warm to room temperature. In other systems this can tend to decompose the materials involved.
It is also necessary to purify the crude product directly after the work up procedure to ensure decomposition of the product doesnt take place.
All glassware should be flame dried and needles and syringes should be purged with nitrogen to ensure good yields.
The scale of this reaction works well on moderately scaled reactions i.e 0.1-0.25 g of the enamide, however attempts at scaling down the reaction (i.e 10 mg) have given disappointing results. It is clear to see if the reaction has gone to completion as the addition of a solution of the enamide gives a bright yellow solution.
Data
1H NMR (300 MHz, CDCl3) 0.72-0.78 (6H, m, CCH3 and (CH2)5CH3) 1.19-1.34 (6H, m, (CH2)3CH3) 1.47-1.54 (2H, m, C(O)CH2CH2) 2.80 (3H, s, NCH3) 2.83-3.00 (2H, m, C(O)CH2) 3.77-3.86 (1H, m, CHCH3) 5.21 (1H, d, J 8.57 CHPh) 7.05-7.28 (5H, m, CHar)
Lead Reference
B.H. Lipshutz, R.S. Wilhelm, J.Am.Chem.Soc, 1981, 103, 7672-7674.
Keywords
Comments
For another example of a copper mediated conjugtae addition see [[35|Page 35]]
By Stephen Caddick on August 23, 2001