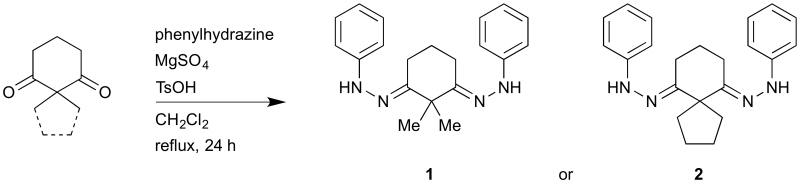
Bis(phenylhydrazones) derived from 2,2-dialkyl cyclohexane-1,3-diones
SyntheticPage 815
DOI:
Submitted: December 4, 2016, published: May 7, 2017
Authors
Jacob Capek (Capek0064@gmail.com)
Kenneth Tritch (trit0026@umn.edu)
Nathan Klein (nklein@mit.edu)
Wayland Noland (nolan001@umn.edu)
A contribution from
Chemicals
synthesis:
2,2-Dimethylcyclohexane-1,3-dione ([Ref. A: One of 2 other Synthetic Pages submitted today])
Spiro[4.5]decane-6,10-dione (Ref. 1)
Phenylhydrazine (97%; Aldrich)
Magnesium sulfate (anhydrous, 97%; Aldrich)
Sodium bicarbonate (ACS grade; Fisher Chemical)
Toluene-4-sulfonic acid monohydrate (98%; EM Science)
Dichloromethane (ACS grade; Fisher Chemical)
Ethyl acetate (ACS grade; Fisher Chemical)
Hexane (ACS grade; Fisher Chemical)
Pentane (ACS grade; Fisher Chemical)
TLC visualization:
(2,4-Dinitrophenyl)hydrazine (97%; Aldrich)
Ethanol (190 proof, ACS/USP grade; Pharmco-AAPER)
Sulfuric acid (95–98%; VWR Analytical)
Procedure
Ketone (2–3 g), CH2Cl2 (80 mL), and MgSO4 (2.0 eq.) were combined in a round-bottomed flask, with magnetic stirring. Phenylhydrazine (2.0 eq.), and then toluene-4-sulfonic acid monohydrate (4–7 mol%) were added. The resulting orange-colored mixture was refluxed for 24 h in an N2 atmosphere, allowed to cool to room temperature, and then filtered. The filtrate was washed with NaHCO3 solution (aq., sat., 30 mL), and then brine (30 mL). The organic portion was dried with MgSO4, filtered, and then concentrated on a rotary evaporator. The resulting brown residue was recrystallized from toluene, washed with pentane, and then dried under vacuum (0.1 mm Hg, 97 ºC, 4 h), giving yellow flakes.
Author Comments
Precautions should be taken to avoid any potential human exposure to phenylhydrazine, which has numerous chronic toxicities (Ref. 2).
By concentration of the filtrate, the product can be carried forward (e.g., for Fischer indolization, Ref. 3) at approx. 85 mass% purity, in 90 to 95% yields, with a shelf life limited to several days. The principal impurities are CH2Cl2, phenylhydrazine, the corresponding monophenylhydrazone, recovered ketone, and toluene-4-sulfonic acid.
The product is moderately air- and water-sensitive. The product mixture is likely to darken visibly during the bicarbonate and brine washes. Samples stored in nitrogen-flushed, capped vials gradually became dark and tarry over 6–12 months. Thus, Schlenck techniques are recommended for storage longer than several months.
Oxygenated solvents are very difficult to remove from the product. The methanol peaks in the NMR spectra are from attempted recrystallizations from methanolic mixtures.
The reaction rate and extent were improved by placing the MgSO4 directly into the reaction mixture, versus use of a Dean-Stark apparatus with toluene, or passing refluxing solvent over a drying agent.
The starting ketones have no strong 254 nm (U.V. light) chromophores. It is advisable to use (2,4-dinitrophenyl)hydrazine (DNPH) solution to assist with thin-layer chromatography visualization (Ref. 4). Our visualization solution was prepared by dissolving DNPH (12 g) in ethanol (200 mL) and water (80 mL), followed by the careful addition of sulfuric acid (60 mL). Care should be taken not to allow solidified DNPH to accumulate, especially on surfaces that are exposed to friction, such as the threads of jar lids. Crystallized DNPH can be shock-sensitive.
Data
C20H24N4 (1)
Yield = 62%.
M.p. 134–136 ºC
Rf = 0.67 on Al2O3 in 1:1 ethyl acetate:hexane
1H NMR (300 MHz, CD2Cl2) δ 7.24 (dd, J = 8.6, 7.3 Hz, 4H, meta-CH), 7.09 (dd, J = 8.7, 1.2 Hz, 4H, ortho-CH), 7.06 (s, 2H, NH), 6.83 (tt, J = 7.5, 1.2 Hz, 2H, para-CH), 2.47 (t, J = 6.8 Hz, 4H; C4, C6), 2.10 (p, J = 7.0 Hz, 2H; C5), 1.53 (s, 6H, CH3).
13C{1H} NMR (126 MHz, CD2Cl2) δ 157.4 (2C, C=N; C1, C3), 153.0 (2C, ArC–N; ipso-C), 134.7 (4C, ArCH; meta-C), 124.8 (2C, ArCH, para-C), 118.8 (4C, ArCH; ortho-C), 51.8 (1C, Cq; C2), 32.6 (2C, CH3), 29.5 (2C, CH2; C4, C6), 24.6 (1C, CH2; C5). Atom assignments are based on HMBC analysis.
ESI-MS m/z calcd for [M–H]– 319.1928, found 319.1930.
IR (KBr, cm–1): ν(N–H) 3348, 1706; ν(C–H), 3051, 2981, 2894; ν(C=N) 1601.
C22H26N4 (2)
Yield = 44%.
M.p. 121–124 ºC
Rf = 0.65 on Al2O3 in 1:1 ethyl acetate:hexane
1H NMR (300 MHz, CD2Cl2) δ 7.23 (dd, J = 8.6, 7.3 Hz, 4H, meta-CH), 7.08 (dd, J = 8.7, 1.2 Hz, 4H, ortho-CH), 7.02 (s, 2H, NH), 6.82 (tt, J = 7.5, 1.2 Hz, 2H, para-CH), 2.44 (t, J = 6.7 Hz, 4H; C7, C9), 2.30–2.19 (m, 4H; C1, C4), 2.09 (p, J = 6.6 Hz, 2H; C8), 1.84–1.79 (m, 4H; C2, C3).
13C{1H} NMR (75 MHz, CD2Cl2) δ 150.3 (2C, C=N; C6, C10), 146.8 (2C, ArC–N; ipso-C), 129.6 (4C, ArCH; meta-C), 120.0 (2C, ArCH, para-C), 113.4 (4C, ArCH; ortho-C), 57.2 (1C, Cq; C6), 35.9 (2C, CH2; C1, C4), 26.2 (2C, CH2; C2, C3), 24.9 (2C, CH2; C7, C9), 18.3 (1C, CH2; C8). Atom assignments are based on HMBC analysis.
ESI-MS m/z calcd for [M+Na]+ 369.2050, found 369.2048.
IR (KBr, cm–1): ν(N–H) 3347, 1701; ν(C–H), 3051, 2952, 2865; ν(C=N) 1601.
Lead Reference
Oppolzer, W.; Zutterman, F.; Bättig, K. Helv. Chim. Acta 1983, 66, 522–533, esp. compound 24.
Other References
1. Miah, M. A. J.; Snieckus, V. J. Org. Chem. 1985, 50, 5438–5440.
2. Karbownik, M.; Reiter, R. J.; Garcia, J. G.; Tan, D.-X. Int. J. Biochem. Cell Biol. 2000, 32,
1045–1054.
3. Bergman, J.; Norrby, P.-O.; Tilstam, U.; Venemalm, L. Tetrahedron 1989, 45, 5549–5564, esp.
compound 7.
4. Leonard, J.; Lygo, B.; Procter, G. Advanced Practical Organic Chemistry, 2nd ed.; Chapman
& Hall: Glasgow, G64 2NZ, UK, 1995, esp. pp 148–149.
Supplementary Information
Keywords
electrophilic, hydrazones, ketones, substitution