Alkylation of 1-methylimidazole with alkyl chlorides
SyntheticPage 792
DOI:
Submitted: July 29, 2015, published: August 17, 2015
Authors
Jeraime Griffith (j.griffith@imperial.ac.uk)
A contribution from
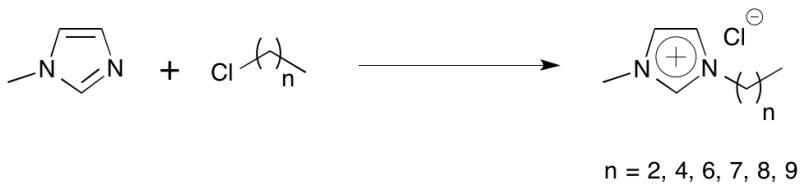
Chemicals
1-Methylimidazole
Activated decolourising charcoal
1-Chloropropane
1-Chloropentane
1-Chloroheptane
1-Chlorooctane
1-Chlorononane
1-Chlorodecane
Celite®
Deionised water
Anhydrous methanol
0.2 micron PTFE filter
Phosphorous pentoxide
Potassium hydroxide
Procedure
The haloalkane (1.1eq) was added dropwise to a stirred 1:1 mixture of 1-methylimidazole (1eq) and anhydrous ethyl acetate at 0°C. After complete addition the mixture was stirred at ambient temperature for 30 minutes before slowly warming to 60°C. Progress was monitored by 1HNMR. At 10-14 days the mixture was cooled to ambient temperature and the upper phase of the biphasic mixture was removed under schlenk conditions. Further anhydrous ethyl acetate was added and the mixture stirred vigorously between a few hours to overnight. The upper phase was then removed and the lower (ionic liquid) phase analysed by 1HNMR. The washing step was repeated until the lower phase was free of starting materials. If necessary, as judged by the colour of the lower phase, activated decolourising charcoal was added and the mixture (in water or methanol) stirred at 50°C overnight then cooled and filtered through a bed of Celite®. Additional filtration through a 0.2 micron PTFE filter removes any residual particulates. The filtrate was then concentrated under reduced pressure and dried under high vacuum at 55°C to afford clear viscous oils.
Author Comments
2. Oven dry of glassware prior to reaction. Ensure glass joints are tight-fitting (avoid grease) to ensure an inert atmosphere.
3. Higher temperatures (shorter reaction time) leads to a coloured product and tedious purification.
4. 1-Propyl-3-methylimidazolium chloride was obtained as a viscous oil. On prolonged standing it presented as a low melting waxy solid.
5. It is believed that the others remained as (supercooled) ionic liquids in the absence of a seed crystal or nucleation sites.
Data
1-Pentyl-3-methylimidazolium chloride - Clear colourless viscous oil (56g, 47%). 1HNMR (400 MHz, DMSO-d6) δH 9.29 (1H, s), 7.82 (1H, s), 7.74 (1H, s), 4.17 (2H, t), 3.86 (3H, s), 1.79 (2H, quintet), 1.34-1.19 (4H, m), 0.87 (3H, t); 13CNMR (101 MHz, DMSO-d6); m/z 153 ([PentMIM]+, 100%), 55, 43, 41, 37, 35 (Cl-, 100%).
1-Heptyl-3-methylimidazolium chloride - Treated with activated decolourising charcoal. Colourless to pale yellow viscous oil (78.7g, 65%). 1HNMR (400 MHz, DMSO-d6) δH 9.39 (1H, s), 7.85 (1H, s), 7.77 (1H, s), 4.18 (2H, t), 3.88 (3H, s), 1.78 (2H, quintet), 1.32-1.21 (8H, m), 0.86 (3H, t); 13CNMR (101 MHz, DMSO-d6); m/z 181 [HeptMIM]+, 100%), 37, 35 (Cl-, 100%).
1-Octyl-3-methylimidazolium chloride - Pale yellow viscous oil (38.7g, 42%). 1HNMR (400 MHz, DMSO-d6) δH 9.28 (1H, s), 7.82 (1H, s), 7.75 (1H, s), 4.17 (2H, t), 3.87 (3H, s), 1.78 (2H, quintet), 1.32-1.20 (10H, m), 0.86 (3H, t); 13CNMR (101 MHz, DMSO-d6) δC 137.22, 124.02, 122.73, 49.12, 36.15, 31.63, 29.91, 28.96, 28.83, 25.97, 22.52, 14.39; m/z 195 [OctMIM]+, 100%), 83, 37, 35 (Cl-, 100%).
1-Nonyl-3-methylimidazolium chloride - Clear colourless viscous oil (80.1g, 65%). 1HNMR (400 MHz, DMSO-d6) δH 9.38 (1H, s), 7.84 (1H, s), 7.77 (1H, s), 4.18 (2H, t), 3.87 (3H, s), 1.78 (2H, quintet), 1.30-1.21 (12H, m), 0.86 (3H, t); 13CNMR (101 MHz, DMSO-d6) δC 137.22, 124.02, 122.73, 49.12, 36.15, 31.70, 29.91, 29.26, 29.07, 28.88, 25.97, 22.54, 14.40; m/z 209 [NonMIM]+, 100%), 37, 35 (Cl-, 100%).
1-Decyl-3-methylimidazolium chloride - Clear colourless viscous oil (70.1g, 62%). 1HNMR (400 MHz, DMSO-d6) δH 9.31 (1H, s), 7.82 (1H, s), 7.75 (1H, s), 4.17 (2H, t), 3.87 (3H, s), 1.78 (2H, quintet), 1.29-1.21 (14H, m), 0.86 (3H, t); 13CNMR (101 MHz, DMSO-d6) δC 137.20, 124.03, 122.73, 49.14, 36.16, 31.75, 29.91, 29.38, 29.31, 29.14, 28.87, 25.98, 22.55, 14.40; m/z 223 [DecMIM]+, 100%), 83, 43, 41, 37, 35 (Cl-, 100%).
Supplementary Information
Keywords
addition, Alkanes, alkyl/alkenyl/aryl halides, amines, heterocyclic compounds, Ionic liquid, nucleophilic, salts