Mechanochemical bromination of 2-naphthol
SyntheticPage 789
DOI:
Submitted: July 17, 2015, published: July 21, 2015
Authors
Marco Santos (santosm@berea.edu)
Nicholas Marshall (nicholas_marshall@berea.edu)
Tyler Hutsell (hutsellt6@gmail.com)
A contribution from
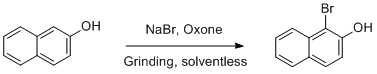
Chemicals
2-Naphthol, 99%, Aldrich
Sodium bromide
Potassium monopersulfate, triple salt with potassium bisulfate and potassium sulfate ("Oxone," Alfa Aesar)
Dichloromethane
n-heptane, HPLC grade, Fisher ScientificProcedure
0.58g (4 mmol) of 2-naphthol, 0.41g (4 mmol) of sodium bromide and 1.225g (2 mmol) of oxone were placed in a mortar and pestle. The reagents were ground together ca. 1-2 minutes until they became a fine powder, then transferred into a plastic reagent bottle with grinding media. (See Notes) The bottle was placed inside a rock tumbler container and tumbled for five hours or overnight. Dichloromethane (ca. 10 mL, see Notes) was added to the container, the container was sealed and vigorously shaken by hand, and the liquid was gravity filtered into a 100 mL round bottom flask through a fluted paper. The dichloromethane wash was repeated a total of 3 times, and the resulting dark brown-red solution was reduced to a solid by rotary evaporation. The resulting crude 1-bromo-2-naphthol is a dark brown solid showing flecks of iridescent color, approximately 0.7g. (See Notes)
This product was crystallized from boiling n-heptane by transferring the solid to a 125 mL Erlenmeyer flask and adding ca. 10 mL of heptane, then heating to boiling using a heat gun. The hot solution was separated from the dark oily residue by decanting into a beaker pre-cooled in an ice bath, and the resulting clusters of beige, needlelike crystals were collected in a Büchner funnel and washed with ice-cold n-heptane, yielding 0.383 g (1.72 mmol) of 1-bromo-2-naphthol, 43% yield.Author Comments
-
This reaction was developed as part of a research project focused on developing green halogenations in the solid state. Many examples exist of using high-speed (30 Hz) ball mills for reactions, but we wanted to develop a procedure that worked with inexpensive and easily available equipment but which did not require continuous manual grinding like our previous report in Synthetic Page 753
-
An inexpensive rock tumbler suitable for the procedure is available at the time of this writing from Harbor Freight (http://www.harborfreight.com/3-lb-rotary-rock-tumbler-67631.html) for USD $41.99. Simple quartz or granite tumbled gravel is suitable for grinding media; for the 4 mmol scale shown, we used 3-5 stones of about 2 cm diameter. A reused wide-mouthed reagent bottle, 100g size, from Sigma-Aldrich was the reaction vessel. It is also possible to use a 50 mL centrifuge tube and steel BB shot or ball bearings as the vessel and grinding media respectively.
-
Dichloromethane is not a green solvent and is used here for validation of the procedure. Ethyl acetate or dimethyl carbonate can be substituted without any difficulties.
-
The crude product contains ca. 15% of a m/z 248/250 (1:1) impurity which is likely 3-bromo-2-hydroxy-1,4-naphthoquinone, a byproduct from direct oxidation of the brominated naphthol.
-
The reaction in the tumbler can be used to brominate a variety of activated aromatic compounds including aryl ethers and anilides.
Data
ATR-FTIR (cm-1): 3262 (s, br), 3064 (w), 1631 (m), 1603 (w), 1500 (m), 1433 (m), 1347 (s), 1302 (s), 1235 (m), 1209 (w), 1145 (w), 986 (m), 930 (m), 844 (s), 744 (s), 726 (w)
GC-MS m/z (% relative intensity, ion): 223.9 (98, M+2), 222.0 (100, M+). tr of 1-bromo-2-naphthol product on a Restek 30m RXI-17 fused silica column (see Temperature Program below) was 9.53 minutes.
1H NMR (CDCl3, δ vs. TMS, 300 MHz): 8.03 (d, 1H) 7.77 (dd, 2H) 7.57 (t, 1H) 7.39 (t,1H) 7.26* (d, 1H) 5.91 (s, br, 1H)
* Overlap with CHCl3
Lead Reference
Other References
Supplementary Information
Keywords
alkyl/alkenyl/aryl halides, aromatics/arenes, electrophilic, green, oxidation, solid-state, solventless, substitution