Syn-dioxygenation of 7-methoxy-4-methyl-1,2-dihydronaphthalene
SyntheticPage 785
DOI:
Submitted: June 9, 2015, published: June 28, 2015
Authors
Ravindra Phatake (ravirajphatake@gmail.com)
A contribution from
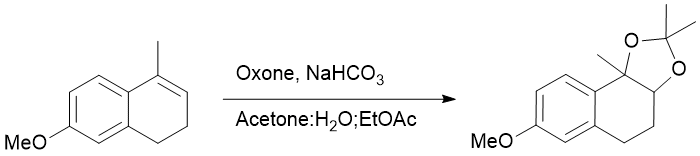
Chemicals
Oxone (2KHSO5.KHSO4.K2SO4)
Sodium bicarbonate (NaHCO3)
Acetone
Ethyl acetate
Water
Procedure
Author Comments
Other benzo-fused olefins like substituted indenes and dihydronaphthalenes work very well.
Reaction condition tolerates different function groups like -Chloro, -Bromo, -azides, etc.
Other than benzo-fused olefins epoxidation is the main event.
Data
1H NMR (500 MHz, CDCl3): δ = 0.97 (s, 3H), 1.41 (s, 3H), 1.54 (s, 3H), 1.94 (td, J = 5.0, 13.4 Hz, 1H), 2.25 (d, J = 13.4 Hz , 1H), 2.58 (dd, J = 5.0, 16.4 Hz, 1H), 3.06 (ddt, J = 5.0, 12.2 Hz, 1H), 3.77 (s, 3H), 4.13 (m, 1H), 6.56 (s, 1H), 6.79 (d, J = 8.5 Hz, 1H), 7.30 (d, J = 8.5 Hz 1H); 13C-NMR (125 MHz, CDCl3): δ = 24.1 (CH2), 24.3 (CH2), 27.3 (3CH3), 55.12 (CH3), 79.0 (C), 79.1 (CH), 107.8 (C), 112.0 (CH), 113.1 (CH), 129.1 (CH), 132.7 (C), 136.5 (C), 158.3 (C) ppm; GC-HRMS (+EI): calcd. for C15H20O3+ 248.1407, found 248.1425.
Lead Reference
Keywords
alkenes, hydroxylation, oxidation, Oxone