N-alkylation in water
SyntheticPage 777
DOI:
Submitted: March 24, 2015, published: March 27, 2015
Authors
Ramesha Ramakrishna (ramesha63@hotmail.com)
A contribution from
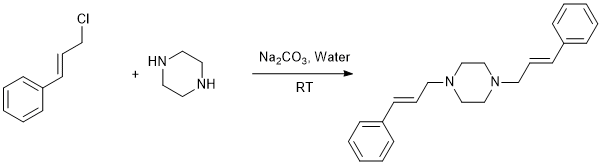
Chemicals
Hydrochloric acid, commercial, about 35%
Piperazine anhydrous, Min 98%, Commercial
Sodium carbonate, LR grade, SD Fine Chem, India
Procedure
1,4-Dicinnamyl piperazine (2): To a 1-L four necked flask carrying a mechanical stirrer, thermometer pocket and an addition flask, water (500 mL) was charged followed by anhydrous piperazine (300 g, 3.48 mole) and sodium carbonate (106 g, 1 mole) . Freshly prepared cinnamyl chloride (300 g, 1.97 mole) was added to the aqueous piperazine solution over a period of 10 min. During the addition, temperature raised upto 60-65oC. After the complete addition, reaction mixture was stirred for another 2 hours without heating or cooling from the external source. During this time, dicinnamyl piperazine precipitates as white solid. Which was filtered and dried under vacuum to get about 236 g of white dicinnamyl piperazine (74.2%).
Preperation of cinnamyl chloride: Cinnamyl alcohol ( 200 g) was added to conc HCl (400 mL) over a period of 5-10 min and stirred at 50-55 0C for 45 min. TLC (2:8, ethyl acetate: petroleum ether) of the reaction mixture shows absence of cinnamyl alcohol and prominent spot at Rf value 0.9. Cool to RT and dilute with water seperate HCl layer and wash the organic layer twice with water to et Cinnamyl chloride as light yellow colore liquid (~ 210 g, 92.6%)
Author Comments
Cinnamyl chloride shold be prepared fresh. Should be used with in 6 h of preperation.
Hydrochloric acid concentration should be above 33% for conversion to chloride.
Addition of 2% tetrabutyl ammonium chloride (PTC) gives similar yield. But the reaction is more exothermic.
Data
Lead Reference
Supplementary Information
Keywords
alcohols, Alkanes, alkyl/alkenyl/aryl halides, alkylation, amines, cinnamyl, piperazine, substitution, water