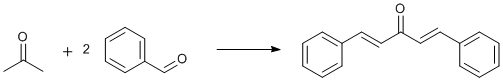
Aldol condensation of benzaldehyde and acetone
SyntheticPage 771
DOI:
Submitted: December 19, 2014, published: December 21, 2014
Authors
Jean-Claude Bradley
Matthew McBride (mcbridemj@gmail.com)
A contribution from
Chemicals
Benzaldehyde (Sigma Aldrich, 98%)
Acetone (Sigma Aldrich, 99%)
Sodium Hydroxide (NaOH)
Ethanol
Distilled Water
Acetone (Sigma Aldrich, 99%)
Sodium Hydroxide (NaOH)
Ethanol
Distilled Water
Procedure
A solution of NaOH (0.902 g, 22.55 mmol, 1.6 eq) in distilled water (40 mL) at room temperature was added to a stirred solution of benzaldehyde (4.361 g, 41.13 mmol, 2.9 eq) in ethanol (40 mL). Acetone (0.833 g, 14.36 mmol, 1.0 eq) was added to the reaction mixture. The reaction mixture was then stirred at room temperature for 30 minutes. The precipitated product was recovered by suction filtration, washed 1x with 2-3mL of 1:1 ethanol/water, dried over suction for 25 minutes, and recovered (3.044 g, 90.6%) as yellow crystals (mp 103-104°C).
Author Comments
•This procedure produced pure trans-dibenzalacetone (verified by NMR spectroscopy) without having to recrystallize. However, the measured melting point of the product (103-104°C) is less than the literature value (110-112°C). If needed, the product may be recrystallized from ethanol.
•The benzaldehyde must be dissolved in the ethanol prior to addition to the water solution.
•The benzaldehyde must be dissolved in the ethanol prior to addition to the water solution.
Data
1H NMR Spectrum (500 MHz, CDCl3): δ 7.72 (d, J = 16.3 Hz, 2H), δ 7.58 (m, 4H), δ 7.38 (m, 6H), δ 7.06 (d, J = 15.5 Hz, 2H)
Lead Reference
B. L. Hawbecker, D. W. Kurtz, T. D. Putnam, P. A. Ahlers, G. D. Gerber, Journal of Chemical Education 1978 55(8), 540 http://dx.doi.org/10.1021/ed055p540
Other References
M. J. McBride, J. C. Bradley. UsefulChem, Experiment 284. http://usefulchem.wikispaces.com/EXP284
Keywords
Aldol Condensation