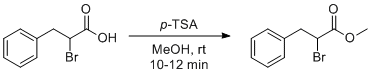
Esterification of 2-bromo-3-phenylpropanoic acid
SyntheticPage 765
DOI:
Submitted: September 14, 2014, published: September 22, 2014
Authors
Ravindra Phatake (ravirajphatake@gmail.com)
A contribution from
Chemicals
2-Bromo-3-phenylpropanoic acid
p-Toluenesulfonic acid (PTSA or pTsOH)
Sodium bicarbonate (NaHCO3)
Methanol
Procedure
To a well-stirred mixture of 2-bromo-3-phenylpropanoic acid (10.0 g, 43.65 mmol) in methanol (100 mL) at about 0 oC was added p-Toluenesulfonic acid (1.5 g, 8.73 mmol) in portion wise manner. The mixture was then stirred at room temperature for 10-12 min. The reaction mixture was added about 20% sodium bicarbonate solution (50-60 mL). Excess methanol from reaction mixture was evaporated on rota evaporator and remaining reaction mass was extracted in ethyl acetate (100 mL × 2). The combined organic layer dried over sodium sulphate and concentrated under reduced pressure to obtain the product methyl 2-bromo-3-phenylpropanoate (10.2 g, 96 %). All examples gave NMR pure Products (95-98 %).
Author Comments
The reaction seems to be ideal for both large and small scale (10 g- 50mg). Another alcohol (Solvent) like ethanol also works well but taking more time to complete the reaction.
Data
1H NMR (500 MHz, CDCl3): δ = 3.30 (dd, J = 7.1, 14.1 Hz, 1H), 3.53 (dd, J = 8.5, 14.1 Hz, 1H), 3.76 (s, 3H), 4.46 (dd, J = 7.1, 8.5 Hz, 1H), 7.24–7.26 (m, 2H), 7.28–7.31 (m, 1H), 7.33–7.36 (m, 2H); 13C-NMR (125 MHz, CDCl3): δ = 41.1 (CH2), 45.1 (CH), 52.9 (CH3), 127.3 (CH), 128.6 (2CH), 129.1 (2CH), 136.7 (C), 169.9 (C) ppm.
Lead Reference
C. Vieville, Z. Mouloungui, A. Gaset Ind. Eng. Chem. Res. 1993, 32, 2065
Other References
N. H. Isaiah, R. Subbarao, J. S. Aggarwal J. Chromatogr., 1969, 43, 519
Keywords
carboxylic acids, esterification, esters