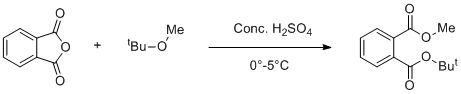
Esterification of Phthalic anhydride with tert-butyl methyl ether (TBME)
SyntheticPage 760
DOI:
Submitted: August 21, 2014, published: September 12, 2014
Authors
M. Bhagavan Raju (mbhagavanraju@gmail.com)
Pankaj Dawar (dawar.pankaj@gmail.com)
Ramesha A. Ramakrishna (ramesha63@hotmail.com)
A contribution from
Chemicals
Phthalic anhydride (98%, Sigma-Aldrich)
tert-butyl methyl ether (anhydrous, 99.8%, Sigma-Aldrich)
Concentrated Sulfuric Acid (98%, Sigma-Aldrich)
Dichloromethane (Merck)
Sodium carbonate (Sigma-Aldrich)
Procedure
Author Comments
It is important to do reaction below 0-5°C, probably ~0°C. Higher temperature will give Dimethyl phthalate.
Phthalic anhydride should be > 98% pure. If it is contaminated with phthalic acid reaction will not proceed well.
Data
IR (cm-1): 1851.66 ([C=O]),1752.23 ([C=O]), 172.05 (ester).
1H NMR ( CDCl3; 400MHz ): δ 8.02-7.26 ( m, 4H, ArH ), 3.90 ( s, 3H, OCH3 ), 1.57 ( m, 9H, -(CH3)3 ).
13C NMR ( CDCl3; 100 MHz ): 168.2, 166.5, 135.9, 133.5, 131.8, 131.0, 130.8, 130.4, 128.8, 128.7, 125.5, 82.04, 52.5, 27.9.
Lead Reference
Keywords
Esterification, esters, ethers