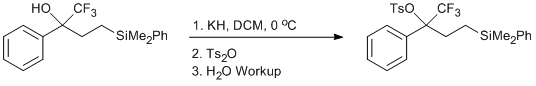
Tosylation of a Trifluoromethyl Carbinol
SyntheticPage 755
DOI:
Submitted: July 10, 2014, published: August 11, 2014
Authors
Christopher Kelly (christopher.b.kelly@uconn.edu)
Leon Tilley (ltilley@stonehill.edu)
Michael Mercadante (michael.mercadante@uconn.edu)
Nicholas Leadbeater (nicholas.leadbeater@uconn.edu)
A contribution from
Chemicals
Potassium Hydride (30 wt % dispersion in mineral oil, Sigma Aldrich)
Dichloromethane (CHROMASOLV®, for HPLC, ≥99.8% Sigma Aldrich)
p-Toluenesulfonic Anhydride (Oakwood Products)1
Procedure
In a glove box under argon, a suspension of KH in mineral oil was washed multiple times with pentane and allowed to dry.2 A flame-dried 1000 mL 3-necked, round-bottomed flask equipped with a stir bar, nitrogen inlet adapter, and two septa, was placed in the glove box. The now dry KH (3.29 g, 0.082 mol, 2 equiv) was placed in the flask and sealed with the septa and inlet adapter and removed from the glove box. The flask was then placed under a nitrogen atmosphere via the nitrogen inlet adapter. DCM (410 mL) was added to the flask via syringe. The reaction mixture was cooled to 0 oC in an ice-water bath for approximately 10 minutes while stirring. To this chilled mixture was added a solution of the CF3 alcohol (14.0 g, 0.041 mol, 1 equiv) dissolved in DCM (41 mL) and dropwise via syringe. Gas evolution and foaming was observed, consistent with deprotonation of the trifluoromethyl alcohol. After five minutes, the ice bath was removed and the reaction mixture allowed to stir at room temperature for 1 hour. The mixture was then placed back in the ice-water bath and allowed to cool for 10 minutes. Under a stream of nitrogen, the septum was removed and all at once, Ts2O (20.25g, 0.062 mol 1.5 equiv) was added. The mixture was removed from the ice bath and allowed to stir for 1 hour, at which time it became thicker and an off-white precipitate was observed to form.
At this point, 400 mL of pentane was syringed into the reaction mixture. Deionized water (50 mL) was then added very slowly to the reaction mixture, to ensure that gas evolution was not too rapid.3 Caution: it is important that water be added slowly so as not to generate too much heat and avoid the possibility of fire. The reaction mixture was left to stir for 15 minutes. During this time, the solution became clear indicating the quench was complete. The contents of the reaction flask were then transferred into a separatory funnel and the aqueous layer removed. The organic layer containing the tosylated product was transferred to an Erlenmeyer flask and dried with Na2SO4. The solvent was then removed via rotary evaporation in a 30 oC water bath and then placed under a high vacuum to remove residual solvent to give the desired product as a thick, yellow oil (20.102 g, 99%)4,5,6.
Author Comments
2. This usually takes 10-15 minutes. It will usually become light grey and fluffy when dry.
3. A vent needle is highly advisable, espcially on this scale or greater.
4. Because of the viscosity of the resulting product, trace amounts of pentane can never be fully removed.
5. These toysylates can be stored but not for extend periods as they will decompose. They should be subjected to solvolysis relatively soon after preparation. The solvolysis protocol will be disclosed in a forthcoming Chemspider Synthetic page.
6. The correpsonding mesylates of these alcohols can be prepared using a less entailed protocol. They can equally be used for solvolysis. The protocol for preparing mesylates will be disclosed in a forthcoming Chemspider Synthetic page.
Data
Lead Reference
Other References
Kelly, C. B.; Colthart, A. M.; Constant, B.D.; Corning, S.R.; Dubois, L. N. E.; Genovese, J. T.; Radziewicz, J. L.; Sletten, E. M.; Whitaker, K. R.; Tilley, J. J.Org. Lett. 2011, 13, 1646.
Keywords
alcohols, Leaving Groups, nucleophilic, Sulfonate Esters, Toyslate