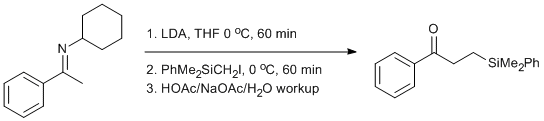
Alkylation of a Cyclohexylimine with (iodomethyl)dimethylphenylsilane
SyntheticPage 754
DOI:
Submitted: July 10, 2014, published: August 11, 2014
Authors
Christopher Kelly (christopher.b.kelly@uconn.edu)
Leon Tilley (ltilley@stonehill.edu)
Michael Mercadante (michael.mercadante@uconn.edu)
Nicholas Leadbeater (nicholas.leadbeater@uconn.edu)
A contribution from
Chemicals
Lithium Diisopropylamide (2.0 M in THF/heptane/ethylbenzene, Sigma Aldrich)
(Iodomethyl)dimethylphenylsilane (Prepared in a forthcoming Chemspider Synthetic Page)
Tetrahydrofuran (CHROMASOLV® Plus, for HPLC, ≥99.9%)
Acetic Acid (Glacial, Certified ACS Plus, Fisher)
Sodium Acetate (ReagentPlus®, ≥99.0% Sigma Aldrich)
Procedure
To a 250 mL round bottom flask equipped with stirbar was added N-(1-Phenylethylidene)cyclohexanamine (13.0 g, 0.064 mol 1 equiv) and THF (18 mL). The flask was sealed with a rubber septum, placed under a nitrogen atmosphere via a nitrogen inlet needle, and stirred via a magnetic stir plate. The reaction mixture was cooled to 0 oC in an ice-water bath for approximately 10 minutes. To this chilled mixture was added a 2 M solution of lithium diisopropylamide1 in THF/ethylbenzene/heptane (35.2 mL, 0.0704 mol, 1.1 equiv) dropwise via a 60 mL syringe.2 The reaction mixture was allowed to stir for 1 hour at 0 oC.3 After this time, (iodomethyl)trimethylsilane (19.44 g, 0.0704 mol, 1.1 equiv) was added dropwise via a 30 mL syringe. The reaction mixture was allowed to stir for 1 hour at 0 oC. After this time, the septa was removed and 65 mL of an acetate buffer (32.5 g NaOAc•3H2O, 65 mL HOAc, 65 mL H2O) was added all at once. Initially a precipitate formed but dissolved after stirring for 10 minutes at 0 oC. The reaction mixture was transferred to a large separatory funnel and diluted with pentane4 (≈400 mL) and water (≈100 mL). The layers were separated and the aqueous layer was extracted three times with pentane (≈50 mL each). The organic layers were combine and were washed with a saturated sodium bicarbonate solution in a separatory funnel (CAUTION: The pentane layer contains some acetic acid. Quenching this with the bicarbonate solution releases CO2 gas and hence the separatory funnel must be vented often to avoid pressure build-up). The washes were continued until no more gas was evolved (roughly three washes of 50 mL each). The organic layer was washed with brine (≈ 200 mL) and dried with Na2SO4. The solvent was removed in vacuo and then purified via vacuum distillation (b.p. 140-143 oC @ 0.10 mmHg) giving the ketone (15.24 g, 88%) as a clear, light yellow oil.
Author Comments
1. The use of fresh LDA is recommended. Bottles stored for extended periods of time have a tendency to lose activity.
2. This can be performed relatively fast
3. The reaction will, over time, turn yellow and form a precipitate which can cause the solution to solidify. This has no effect on the reaction success.
4. We initially used ether in these extractions but found that we could mitigate the uptake of acetic acid by switching to pentane without compromising yield. Hexanes can be used as well although pentane is easier to remove upon rotary evaporation.
Data
Lead Reference
Other References
Fleming, I.; Patel, S. K.; Urch, C. J. Chem. Soc. Perk. Trans. I 1989, 115.
Keywords
Alkanes, Alkylation, Imines, ketones, Organosilicon