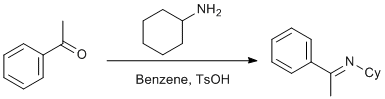
Condensation of cyclohexylamine and acetophenone
SyntheticPage 752
DOI:
Submitted: July 10, 2014, published: August 11, 2014
Authors
Christopher Kelly (christopher.b.kelly@uconn.edu)
Leon Tilley (ltilley@stonehill.edu)
Michael Mercadante (michael.mercadante@uconn.edu)
Nicholas Leadbeater (nicholas.leadbeater@uconn.edu)
A contribution from
Chemicals
Acetophenone (ReagentPlus®, 99% Sigma Aldrich)
p-Toluenesulfonic Acid (ACS reagent, ≥98.5% Sigma Aldrich)
Benzene (ACS reagent, ≥99.0% Sigma Aldrich)
Procedure
Author Comments
1. To ensure that the vapor made it to the condenser, the Dean-Stark trap was insulated with a layer of glass wool.
2. Residual cyclohexamine would often distill at lower temperatures (20-30 oC)
3. Use of SiO2 or similar purification procedures is not advisable as the imines are unstable in the presence of acidic media (will revert to the ketone and the amine
4. Long term storage (> 2 weeks) of the imines requires either storage in a dry non-polar solution (e.g. pentane and MgSO4) or storage in a moisture free environment such as a glovebox or desiccator. Exposure to atmospheric conditions will result in slow be steady hydrolysis back to the ketone and amine.
Data
1H NMR (400 MHz, CDCl3) d ppm 1.26 - 1.45 (m, 3 H) 1.56 (qd, J = 12.00, 3.00 Hz, 2 H) 1.65 - 1.74 (m, 3 H) 1.80 - 1.88 (m, 2 H) 2.25 (s, 3 H) 3.49 (spt, J = 4.80 Hz, 1 H) 7.33 - 7.39 (m, 3 H) 7.75 (dd, J = 6.83, 2.73 Hz, 2 H) 13C NMR (125 MHz, CDCl3) d ppm 15.31 (CH3) 25.05 (CH2) 26.01 (CH2) 33.77 (CH2) 60.02 (CH) 126.82 (CH) 128.27 (CH) 129.22 (CH) 142.06 (C) 162.39 (C) GC-MS (EI) 201 ([M]+, 20%), 200 ([M-1]+, 51%), 186 (100%), 172 (19%), 158 (47%), 146 (27%), 144 (26%), 130 (29%), 120 (68%), 117 (17%), 104 (94%), 91 (16%), 77 (37%), 55 (24%), 41 (19%).
Lead Reference
Other References
Watson, J. M.; Irvine, J. L.; Roberts, R. M. J. Am. Chem. Soc., 1973, 95, 3348.
Keywords
amines, condensation, imines, ketones