Ritter reaction and esterification with isobutyl alcohol
SyntheticPage 750
DOI:
Submitted: July 3, 2014, published: July 9, 2014
Authors
M. Bhagavan Raju (mbhagavanraju@gmail.com)
Pankaj Dawar (dawar.pankaj@gmail.com)
Ramesha A. Ramakrishna (ramesha63@hotmail.com)
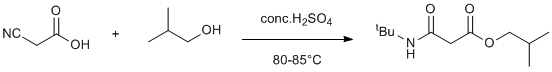
Chemicals
2-cyanoacetic acid (Sigma Aldrich)
Isobutyl alcohol (Sigma Aldrich)
Sulfuric acid (Vetec)
Dichloromethane (Sigma Aldrich)
Procedure
Author Comments
We observed interesting esterification and Ritter reaction product. When isobutyl alcohol is reacted with 2-cyanoacetic acid it gave interesting isobuty ester and tert-butyl amino Ritter product in moderate yield.
To the best of our knowledge and available literature, this is the first example of Ritter reaction of an unactivated alcohol.
Data
IR(cm-1): 3292.49 ([NH]), 1741.72 ([ester]), 1651.67([NH]).
1H NMR (400 MHz, CDCl3): 4.14 (t, 2H, (OCH2) ), 3.97-3.90( m, 1H, CH), 3.29 (s, 2H,CO-CH2), 1.67-1.60 ( m, 2H, CH2), 1.53-1.43 (m, 2H, CH2), 1.41-1.33 (m, 2H, CH3),1.25 (s, 1H, NH), 1.13(s, 2H, CH2), 0.95-0.89 (m, 3H, CH3).
13C NMR (100 MHz,CDCl3) :169.8, 164.2, 65.3, 46.7, 41.2, 30.4, 29.4, 20.2, 19.0, 13.6, 10.2.
HRMS : calc. For C13H14O4Na (M-NH3) :257.0790. Found: 257.0797 (M-NH3).
Lead Reference
Other References
Keywords
alcohols, amides, amination, amino acids, carboxylic acids, esters, ethers