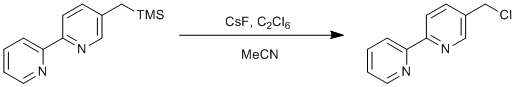
Chlorination fo 5-((trimethylsilyl)methyl)-2,2'-bipyridine
SyntheticPage 749
DOI:
Submitted: June 30, 2014, published: August 12, 2014
Authors
Alan Faulkner (alan.faulkner@warwick.ac.uk)
A contribution from
Chemicals
Hexachloroethane (Aldrich)
CsF (Aldrich)
Acetonitrile (Dried over CaH2 and distilled before use)
Procedure
5-((trimethylsilyl)methyl)-2,2'-bipyridine (1.16 g, 4.5 mmol), hexachloroethane (4.06 g, 9.0 mmol) and caesium fluoride (1.46 g, 9.0 mmol) were suspended in dry acetonitrile (75 ml) and heated to 60˚C for 4 H under an atmosphere of dinitrogen. The reaction mixture was cooled to 25˚C, water (100 ml) was added and the product was extracted in to ethyl acetate (3 x 150 ml). The organic fractions were combined, washed with brine, dried over sodium sulphate, filtered and solvents were removed under reduced pressure. The product was purified by sublimation of any residual hexachloroethane at 60˚C under vacuum to leave the desired product as a beige solid. Yield = 0.84 g, 84 %.
Author Comments
The product itself can also be purified from recystalisation from hot hexane.
Ceasium fluoride is known to be hydroscopic as such once opened it was stored in a nitrogen purge box.
Data
1H NMR (300 MHz, 298 K, CDCl3) δH ppm 8.71-8.66 (2H, m, Py), 8.45-8.38 (2H, m, Py), 7.89-7.79 (2H, m, Py), 7.32 (1H, ddd, 3JHH = 7.4 Hz 3JHH = 4.9 Hz 4JHH = 1.1, Py), 4.65 (2H, s, CH2).
13C{1H} NMR (75 MHz, 298K, CDCl3) δC ppm 156.2 (py), 155.6 (py), 149.3 (py), 149.0 (py), 137.3 (py), 137.0 (py), 133.2 (py), 124.0 (py), 121.3 (py), 121.0 (py), 43.2 (CH2).
MS (ESI) m/z 205.0 [M+H]+.
IR cm-1: 3053 (w), 3008 (w), 2969 (w), 1598 (m), 1587 (m), 1573 (m), 1557 (m), 1493 (w), 1458 (m), 1436 (m), 1392 (m), 1263 (m), 1219 (w), 1196 (w), 1149 (w), 1128 (w), 1090 (w), 1062 (w), 1041 (w), 1028 (m), 993 (w), 935 (w), 904 (s), 857 (w), 834 (m).
Elemental Analysis found (Calculated for C11H9N2Cl) % C 64.56 (64.56), H 4.32 (4.43), N 13.58 (13.68).
Lead Reference
Keywords
aromatics/arenes, Chloroination, electrophilic, heterocyclic compounds