Secondary aromatic amides from anilines
SyntheticPage 746
DOI:
Submitted: May 26, 2014, published: June 11, 2014
Authors
John MacMillan (john.macmillan@temple.edu)
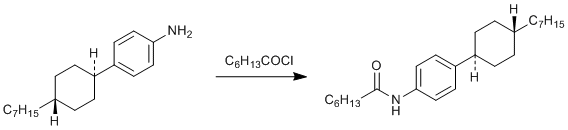
Chemicals
(4-trans-4-Heptylcyclohexyl)aniline, prepared in high yield from the carbamate by basic hydrolysis of Methyl- (p-trans-heptylcyclohexyl)carbamate, see primary reference 1.
Heptanoyl chloride Sigma Aldrich, 99%, 14,724-9
Toluene, Sigma Aldrich ,99.5+ %, A.C.S. Reagent, 17,941-8
Ethanol, 190 proof, Sigma Aldrich , 95+%, 49,351-1
Procedure
Author Comments
CAUTION! Heptanoyl chloride is lachrimatory, irritating and moisture sensitive. Manipulate in a glove box if possible and syringe into a nitrogen blanketed solution. Wear latex gloves. The procedure is applicable for preparing a wide variety of secondary amides from aromatic amines.
The reactions should be run in an efficient fume hood. Small amounts of HCl evolve, which on larger scale can be trapped by addition of equimolar triethlyl amine to the mixture prior to acid chloride.
By entirely analogous reactions other amides were synthesized in high yield by this procedure. See primary and secondary references. Examples:
N-[4-(trans-4-Pentylcyclohexyl)phenyl]heptanamide, m.p. 144-45⁰.
N-[4-(trans-4-Peptylcyclohexyl)phenyl]butanamide, m.p. 142⁰.
Data
m.p. 135-36⁰. I.r. (nujol mull) 3350, 2950, 1660, 1600 cm-1.
Analysis: Calculated for C26H43NO: C, 80.98 H,11.24 N,3.63
Found: C, 81.20 H,11.42 N,3.43
Lead Reference
John H. MacMillan and Mortimer M. Labes, "Low Transition Temperature Liquid Crystalline Amines Incorporating the Trans-1,4-Cyclohexane Ring System", Molecular Crystals and Liquid Crystals, Vol. 55, p 61, (1979).
DOI: dx.doi.org/10.1080/00268947908069791Other References
John H. MacMillan and Mortimer M. Labes, "Low Transition Temperature Liquid Crystalline Amines Incorporating the Biphenyl Ring System", Mol. Crystals and Liquid Crystals Letters, Vol. 56, p51, (1979).
DOI: Link: http://dx.doi.org/10.1080/01406567908071966
John H. MacMillan and Mortimer M. Labes, "Amine Substituted Liquid Crystal Compositions", U.S. Patent 4,293,193, Oct. 6, 1981.
Keywords
acid chloride, amide, amine, amines, aniline, aromatics/arenes, carbamate, carboxylic acids, elimination, nucleophilic, secondary amides, substitution