Nucleophilic Aromatic Substitution of 5-fluoro-1-indanone with morpholine
SyntheticPage 738
DOI:
Submitted: April 21, 2014, published: April 23, 2014
Authors
Adam Glass (glassac@plu.edu)
Drew Huff (huffdb@plu.edu)
Dylan Nehrenburg (nehrenda@plu.edu)
Max Mayther (maythemf@plu.edu)
A contribution from
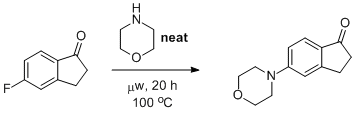
Chemicals
5-fluoro-1-indanone( 99% Sigma Aldrich)
morpholine (99+% Aldrich Chemical Company)
Procedure
The synthesis was performed using standard Schlenk techniques under a nitrogen atmosphere. To a dried and nitrogen flushed glass microwave reaction vial was added 5-flouro-1-indanone (0.101 g, 0.67 mmol). The vial's atmosphere was cycled four times. Morpholine (2.0 mL, 0.023 mol, 3.47 equivs.) was added, The reaction was then sealed and heated to 100 oC for 20 hours under variable watt microwave heating. At the completion of the reaction the crude material was transferred to a 50 mL round bottom flask using 3.5 mL ethyl acetate. Silica and ethyl acetate (4.5 mL) were added to the flask and the solvent was removed under reduced pressure to yield a free flowing dry load. The crude product was then subjected to purification via flash chromatography (75 : 25 ethyl acetate : hexanes) to yield a red solid (144 mg, 98.7%).
We also ran a larger scale reaction (500 mg 1-indanone, 0.0033 mole) to test scale up. We observed a reduced yield isolating 523 mg in a 73% yield.
Author Comments
- The reaction discussed in the lead reference, implementing DMSO as a solvent and a reaction time of three hours, was found to be irreproducible by our group. We observed only trace conversion using this process.
- Basic silica was critical to obtain a reproducible yield and pure product. An basic alumina/silica blend was most effective.
- We used a Biotage Emrys Personal Chemistry Optimizer Microwave Synthesizer for microwave heating.
- Conventional heating was also shown to work, though the reaction times were increased, slightly.
- Trace amounts of the enamine have been characterized as the only minor impurity present.
- Synthesis of 5-morpholino-1-indanone via 5-chloro-1-indanone proved unsuccessful via this approach. The enamine was obtained as the major product.
- We have found that a variable solvent purification works best. Running a nonpolar mix to remove the enamine and they switching to higher polarity to remove the desired product.
Data
1H NMR: (CDCl3) δ ppm 2.59-2.62 (m, 2H), 3.01 (t, J=5.7 Hz, 2H), 3.30 (t, J=5.0 Hz, 4H), 3.83 (t, J=4.7 Hz, 4H), 6.78 (m, 6.78-6.79, 1H), 6.83-6.86 (m, 1H), 7.61 (d, J=8.8 Hz, 1H).
13C NMR: (CDCl3) δ ppm 25.8, 36.3, 47.6, 66.4, 109.7, 114.0, 124.9, 128.2, 155.8, 157.7, 204.9
GCMS EI [M+] Predicted: 217.2, Actual: 217
Lead Reference
Chern, C.-Y.; Yek, Y.-L.; Chen, Y.-L.; Kan, W.-M. J. Chin. Chem. Soc. 2008, 55, 846–853.
Other References
Dinges, J.; Albert, D.H.; Arnold, L.D.; Ashworth, K.L.; et al J. Med Chem. 2007, 50, 2011-2029
Supplementary Information
Keywords
amines, aromatics/arenes, heterocyclic compounds, nucleophilic, substitution