In situ propargyl Grignard addition to vinylic ketone
SyntheticPage 737
DOI:
Submitted: April 9, 2014, published: April 22, 2014
Authors
John MacMillan (john.macmillan@temple.edu)
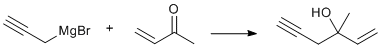
Chemicals
Propargyl bromide (3-bromopropyne), Machester Chemicals, freshly distilled, N34375.
or easily prepared from redistilled propargyl alcohol and phosphorous tribromide.
Magnesium turnings, chip, 99.8%, Sigma-Aldrich, 25,411-8.
Ether, anhydrous, 99.8%,Sigma-Aldrich, unless fresh material, dry just before use over sodium wire or lithium aluminum hydride, 29,608-2.
Mercuric chloride, 98% or better, Sigma-Aldrich, 41,344-5.
Methyl vinyl ketone (3-buten-2-one), 99%, Sigma-Aldrich, 26,954-9, redistill before use.
Ammonium chloride, 99.5%, ACS reagent, Sigma-Aldrich, 21,330-0.
Procedure
An oven-dried one liter three-neck flask was equipped with a mechanical stirrer, heating mantle, dropping funnel, nitrogen inlet, Friedrich water condenser and calcium chloride drying tube. The flask was charged with 0.1 g of mercuric chloride, 24 g (1 mol) of magnesium turnings, 200 ml of dry diethyl ether and 4 g redistilled propargyl bromide. The mixture was stirred, then gently warmed, until reaction commenced after 3 minutes, as evidenced by a gray solution and moderate ether reflux. The flask was then immersed in a dry ice/acetone bath maintained at approximately -20о. A solution of 55 g redistilled propargyl bromide (total 0.50 mol) and 27 g (0.39 mole) 3-buten-2-one in 200 ml dry ether was then added drop-wise, with vigorous stirring, over a 2.5 hour period. The bath was maintained at ~ minus 10о. The mixture was allowed to come to room temperature over one hour and the contents of the flask were poured into a two liter beaker containing a well mixed slurry of ~ 500g crushed ice and ~ 100 g ammonium chloride. The mixture was stirred manually with the mechanical stirrer until two clear layers were present, and all excess magnesium chips and salts had dissolved. The upper ether layer was separated in a separatory funnel and the aqueous layer extracted three times in the separatory funnel with 20 ml ether portions. The combined ether layers were shaken five times with 20 ml deionized water portions and dried over 5g anhydrous magnesium sulfate for one hour. Evaporation of the ether on a steam bath in a fume hood yielded a light yellow oil. Careful fractionation of the crude material in an oil bath under water vacuum pressure ( ~ 80o bath temperature) with a short path distillation column yielded 26.5 g of product in three fractions (62% based on the amount of 3-buten-2-one used), which were >97% pure on both SE-30 and Triton-X-305 columns, and which contained no isomeric internal acetylenic or allenic alcohols. Infrared spectra showed no trace of a characteristic strong allenic stretch at ~1950cm-1 and proton n.m.r. was devoid of peaks due to internal acetylenic and allenic products.
Author Comments
- In order to avoid an internal rearrangement of the propargyl Grignard reagent, a modification of the procedure reported by Sondheimer (secondary reference 1), was utilized. Without this modification, a room temperature standard Grignard synthesis of 1-hexen-5-yne-3-ol (see secondary reference 2), gave an approximate 50/50 mixture of desired product and the rearranged internal alkyne product 1-hexen-4-yn-3ol. This reaction gives yields in the 50-70% range as long as the instructions below are followed.
- As with all Grignard syntheses, glassware should be dried in an oven before use, and the diethyl ether must be 99.9% or dried before use over sodium wire or lithium aluminum hydride.
- Unsaturated carbonyl reactants should be distilled before use.
- Mercuric chloride is toxic, and although used in very small quantities in these reactions latex gloves should be used when manipulating this material.
- Propargyl bromide is toxic and a potent lacrimator, as is 3-buten-2-one. Work in an efficient fume hood.
- Diethyl ether is extremely flammable and open flames must be avoided near this material.
- No trace of product alcohols resulting from rearrangement of propargyl Grignard to internal acetylenic or allenic species could be detected under these conditions.. This synthetic modification is of great utility in preparation of alcohol precursors for acetylenic oxy-Cope rearrangements.
Data
b.p. 54-55°/25 mm, nD22 1.4576, d425 0.8930.
Ir (neat film) 3400(s, OH stretch), 3300(s, alkyn C-H stretch), 3040(m,=C-H stretch),2950(m), 2930(m), 2910(m), 2120(w), 1640(m, C=C stretch), 1450(m), 1410(s), 1370 (s, CH3), 1270(m), 1240(m), 1170(m), 1110(s), 995(m), 920(s), 870(m), 760(m), 750(m), and 650(v.s.) cm-1.
Pmr (60MHz, CDCl3) δ, singlet at 1.40 (3H, Methyl), a doublet at 2.45, (2H, aliphatics), a broad singlet at 3.35,1H, hydroxyl), a doublet of doublets centered at 5.10 and 5.30 (total 2H, terminal vinyl), and a doublet of doublets at 6.10(1H, internal vinyl).
Lead Reference
Other References
1) F. Sondheimer, Y. Amiel, and Y. Gaoni, J. Amer. Chem. Soc., 84, 270-274, (1962).
DOI: 10.1021/ja00861a029
2) A. Viola and J.H. MacMillan, J. Amer. Chem. Soc., 90, 6141, (1968).
DOI: 10.1021/ja01024a035
3) Triple Bond Participation in the Oxy-Cope Rearrangement", John H. MacMillan, Ph.D. Thesis, Northeastern University, 1970, Dissertation Abstracts International, Order Number 72-13,297. http://dissexpress.umi.com/dxweb/results.html?QryTxt=&By=John+Harry+MacMillan&Title=&pubnum=7213297
4) Chemspider depositon: http://www.chemspider.com/Chemical-Structure.29332401.html
Keywords
3-buten-2-one, Acetylene, addition, alcohol, alcohols, aldehydes, alkene, alkenes, alkyne, alkynes, Grignard, halide, ketone, ketones, methyl vinyl ketone, nucleophilic, organometallics, propargyl, propargyl magnesium bromide, vinyl