Dimerization of o-nitro toluene
SyntheticPage 729
DOI:
Submitted: March 21, 2014, published: March 25, 2014
Authors
Ramesha Ramakrishna (ramesha63@hotmail.com)
A contribution from
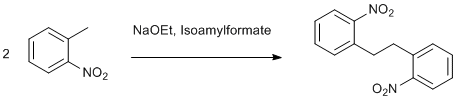
Chemicals
2. Cyclohexane, commercial, 99%
3. Sodium ethoxide, commercial
4. Isoamyl formate, Prepared by refluxing isoamyl alcohol with formic acid and distilling. Purity 98.5%
Procedure
o-Nitro toluene (550 g, 4.01 mol), cylohexane (2000 mL) and isoamylformate (464 g, 4 mol) are charged to a dry 5 Lt -3 necked flask carrying nitrogen inlet, thermometer pocket and mechanical stirrer at room temperature (whole setup should be in cooling tub to give sufficient cooling for 16-18h). With a slow stream of nitrogen, the reaction mass is cooled to about –2 degree centigrade. At this point, slowly add sodium ethoxide (272 g, 4 mol) in lots (about 10-20 g each lot) slowly over a period of 4 hours keeping the temperature below 3 oC. After complete addition, stir the reaction mixture at the same temperature for additional 8-9 h. T this point, one can observe lots of solid formation in the reaction mixture. Add water ( 2 Lt) and adjust the pH to about 6 and filter the solid. Wash the solid with cyclohexane ( 200-250 mL) to get almost 425-450 g o,o-dinitro bibenzyl. This can be dried at 60-70 oC for 6 h to get about 350-360 g product (64% ). This is almost pure by NMR. If required this can be crystallized by acetone to get light yellow product.
Author Comments
2. Quality of sodium ethoxide is important. One can also use commercially avalable sodium tert butoxide.
3. Reaction should be carried out at about 0 degree . Hiigher temperature gives dark oily mass. One cannot recover starting material from this oily mass.
4. Nitrogen should be purged in such a way that it should not carry away all cyclohexane. If required, one can add additional cyclohexane to makeup for the loss.
Data
Melting pont : 120-123 oC
1H NMR (CDCl3, 300 MHz): δ 7.95 (d,2H ), 7.56-7.52 & 7.43-7.36 (2 m,6H), 3.25 (s, 4H)
13C NMR (CDCl3, 75 MHz): δ 149.17, 135.96, 133.27, 132.44, 127.52, 124.80, 34.38
W. Schindler, US Patent, 1956, 2764580
Supplementary Information
13C NMR (13C NMR.bmp)