Deformylation of indole-3-carboxaldehydes
SyntheticPage 723
DOI:
Submitted: March 6, 2014, published: March 7, 2014
Authors
Rammohan R. Yadav (rammohanryadav@gmail.com)
Sandip Bharate (sbharate@iiim.ac.in)
A contribution from
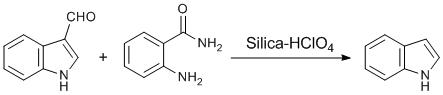
Chemicals
Indole-3-carboxaldehyde (Sigma Aldrich), 97%, 129445
Anthranilamide (Sigma Aldrich), ≥98%, A89804
Silica-HClO4 (preparation provided below)
Acetonitrile (SD Fine Chemicals Ltd), directly used without drying
Procedure
Procedure for deformylation: Silica-perchloric acid (50% w/w, 50 mg) was added to the solution of indole-3-carboxaldehyde (100 mg, 1 mmol) and anthranilamide (94 mg, 1 mmol) in acetonitrile (6 mL). The reaction mixture was allowed to stir for 6 h at reflux temperature (80 °C). The progress of the reaction was monitored by TLC (EtOAc: n-hexane - 1: 9). After completion of the reaction, it was allowed to cool and the catalyst was recovered by filtration. The filtrate was concentrated to get crude product, which after silica gel (mesh 100-200) column chromatography using 10% EtOAc in n-hexane gave indole (65 mg, 80% yield). The product was characterized by comparison of TLC of the product (Rf 0.4 in EtOAc: n-hexane - 1: 9) with commercially available sample (CAS Number 120-72-9; Sigma Aldrich: Catalog no. W259306). It was also confirmed by comparison of NMR data with literature values (Motoyama, Y.; Kamo, K.; Nagashima, H. Org. Lett. 2009, 11, 1345-1348. http://dx.doi.org/10.1021/ol9001366)
Author Comments
-
Deformylation of indole-3-carboxaldehyde proceeds via quinazolinone intermediate (For mechanism, see: Yadav, R. R.; Battini, N.; Mudududdla, R.; Bharate, J. B.; Muparappu, N.; Bharate, S. B.; Vishwakarma, R. A. Tetrahedron Lett. 2012, 53, 2222-2225. http://dx.doi.org/10.1016/j.tetlet.2012.02.079)
Data
Indole: White solid; m.p. 52-53 °C;
1H NMR (CDCl3, 400 MHz) δ ppm: 7.57 (d, J = 11.6 Hz, 1H), 7.44-7.34 (m, 1H), 7.21 (d, J = 2.4 Hz, 1H), 7.11-7.07 (m, 1H), 7.02-6.98 (m, 1H), 6.43 (dd, J = 0.4 & 3.2 Hz, 1H);
IR (CHCl3): 3402, 1456, 1415, 1353, 1247, 1091 cm-1;
GC-MS (EI) m/z (%): 118 (M++H, 10.11), 117 (M+, 100), 90.1 (46.71), 63.2 (12.88).
Lead Reference
Yadav, R. R.; Battini, N.; Mudududdla, R.; Bharate, J. B.; Muparappu, N.; Bharate, S. B.; Vishwakarma, R. A. Deformylation of indole and azaindole-3-carboxaldehydes using anthranilamide and solid acid heterogeneous catalyst via quinazolinone intermediate. Tetrahedron Lett. 2012, 53, 2222-2225. http://dx.doi.org/10.1016/j.tetlet.2012.02.079
Other References
- Hazra, A.; Paira, P.; Sahu, K. B.; Banerjee, S.; Mondal, N. B. Catal. Commun. 2008, 9, 1681–1684. http://dx.doi.org/10.1016/j.catcom.2008.01.028
- Chikvaidze, I. S.; Samsoniya, S. A.; Narindoshvili, T. G.; Kobakhidze, N. V. Chem. Heterocycl. Comp. 2000, 36, 1346. http://dx.doi.org/10.1023/A:1017540005696
Supplementary Information
Keywords
Deformylation, elimination, heterocyclic compounds, heterogeneous catalyst, indole, indole-3-carboxaldehyde, quinazolinone