Hydrolysis of retinyl palmitate
SyntheticPage 718
DOI:
Submitted: February 4, 2014, published: February 5, 2014
Authors
Maksim A. Fomich (mfomich@gmail.com)
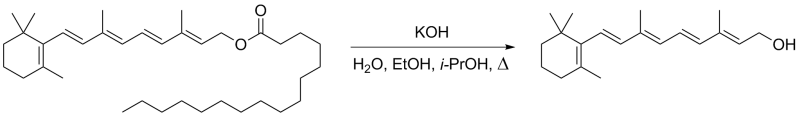
Chemicals
Retinyl palmitate, 85%
Potassium hydroxide, pellets, 85% (Acros Organics)
Ethanol, 95%
Propan-2-ol, 95%
Hexanes, redistilled
Ethyl acetate, redistilled from P2O5
Magnesium sulfate anhydrous , ≥97% (Sigma-Aldrich)
Potassium hydroxide, pellets, 85% (Acros Organics)
Ethanol, 95%
Propan-2-ol, 95%
Hexanes, redistilled
Ethyl acetate, redistilled from P2O5
Magnesium sulfate anhydrous , ≥97% (Sigma-Aldrich)
Procedure
A 10% (w/v) solution of KOH in water (200 mL) was added to a solution of retinyl palmitate (10.5 g, 20.0 mmol) in propan-2-ol (100 mL) and ethanol (100 mL). The reaction mixture was heated to reflux at 90 °C for 75 min, then it was poured into ice water (0.5 L) and extracted with hexanes (2×350 mL). Combined organic layers were subsequently washed with water (300 mL) and brine (300 mL) and dried over anhydrous MgSO4. After filtration and evaporation, the residue was chromatographed on silica (60/200, 250 mL) in EtOAc – hexanes mixture (10 to 20% EtOAc) to yield retinol (3.28 g, 58%) as an orange viscous oil that solidified after several days at –20 °C.
Author Comments
- Special care was taken to protect the flask with reaction mixture from direct sun light as retinol and its derivatives are known to be light-unstable to some extent.
- At the beginning the mixture is heterogeneous but the solid dissolves as the flask is heated.
- Extraction of retinol into hexanes comprises the major difficulty. Because of the presence of sodium palmitate it may take some time for emulsion to break.
Data
TLC: Rf 0.25 (20% EtOAc/hexanes).
1H NMR (CDCl3, 400 MHz) δ 6.61 (dd, J = 15.1, 11.3 Hz, 1Н), 6.28 (d, J = 15.2 Hz, 1Н), 6.19-6.08 (m, 3Н), 5.68 (t, J = 6.8 Hz, 1Н), 4.30 (d, J = 6.9 Hz, 2Н), 2.01 (t, J = 6.1 Hz, 2Н), 1.95 (s, 3Н), 1.86 (s, 3Н), 1.71 (s, 3Н), 1.64-1.58 (m, 2Н), 1.47-1.45 (m, 2Н), 1.02 (s, 6Н).
13C NMR (CDCl3, 101 MHz) δ 137.94, 137.77, 137.01, 136.43, 136.28, 130.20, 130.15, 129.39, 126.88, 125.30, 59.58, 39.73, 34.37, 33.17, 29.07, 21.85, 19.39, 12.84, 12.75.
Lead Reference
H. Tanaka, H. Kagechika, E. Kawachi, H. Fukasawa, Y. Hashimoto, K. Shudo. Base-catalyzed isomerization of retinoic acid. Synthesis and differentiation-inducing activities of 14-alkylated all-trans-, 13-cis-, and 20,14-retro-retinoic acids. J. Med. Chem., 1992, 35 (3), 567–572. http://dx.doi.org/10.1021/jm00081a020
Keywords
esters, hydrolysis