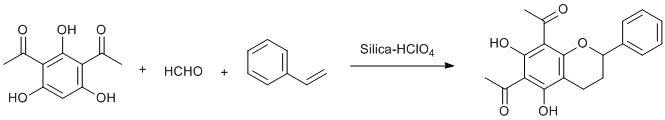
Silica-HClO4 catalyzed tandem Knoevenagel condensation and [4+2]-Diel’s-Alder cycloaddition
SyntheticPage 712
DOI:
Submitted: January 29, 2014, published: February 2, 2014
Authors
Ramesh Mudududdla (ramesh.chem31@gmail.com)
Sandip Bharate (sbharate@iiim.ac.in)
A contribution from
Chemicals
Formaldehyde (SD Fine Chemicals Ltd)
Styrene (Sigma Aldrich), ReagentPlus® ≥99%, S4972
Silica-HClO4 (preparation provided below)
Acetonitrile (SD Fine Chemicals Ltd), directly used without drying
Procedure
Preparation of Silica-HClO4 catalyst: Perchloric acid (1.25 g, as a 70% aqueous solution) was added to the suspension of silica gel (23.75 g, mesh 230–400) in Et2O. The mixture was concentrated and the residue was heated at 100 °C for 72 h under vacuum to afford HClO4–SiO2 as a free flowing powder. http://dx.doi.org/10.1039/B304178F
To the solution of 2,4-diacetyl phloroglucinol (100 mg, 1 mmol) in acetonitrile (10 mL) were added formaldehyde (0.04 mL, 3 mmol), styrene (74 mg, 1.5 mmol) and 50 %w/w silica-HClO4 (50 mg). The mixture was then heated to reflux at 80 °C for 6 h. Completion of the reaction was monitored by TLC (by disappearance of 2,4-diacetyl phloroglucinol on TLC). After completion of the reaction, the mixture was cooled to room temperature and was filtered through filter paper. The filtrate was concentrated on a rotary evaporator to give the crude product as a colourless oil. Purification by silica gel (mesh 100-200) column chromatography using EtOAc: Hexane - 1:1 as eluent gave product as white crystals (84% yield).
Author Comments
- Disubstituted phloroglucinols showed better reactivity compared with mono-substituted followed by non-substituted phloroglucinol (For details, see: Org. Biomol. Chem. 2012, 10, 5143–5150 http://dx.doi.org/10.1039/C2OB25376C).
- The method involves Knoevenagel-type condensation leading to in-situ formation of transient O-quinone methide which further undergoes [4+2]-Diels-Alder cycloaddition with styrene to yield flavan skeleton.
- Key features of the protocol are (a). no protection/deprotection steps required; (b). inexpensive, easy to prepare, non-hazardous, easy to separate from reaction mixture, reusable catalyst and (c). diversity-oriented synthesis.
Data
6,8-Diacetyl-5,7-dihydroxyflavan: Yield: 84%; white crystalline solid; m.p. 101-103 °C
1H NMR (CDCl3, 500 MHz) δ ppm 16.15 (s, 1H), 15.18 (s, 1H), 7.40 (m, 5H), 5.15 (dd, J = 2.3, 10.4 Hz, 1H), 2.76 (m, 1H), 2.72 (s, 3H), 2.66 (m, 1H), 2.53 (s, 3H), 2.24 (m, 1H), 2.02 (m, 1H)
13C NMR (CDCl3, 125 MHz) δ ppm 204.5, 203.4, 171.6, 170.9, 162.6, 140.0, 128.8, 128.5, 127.8, 125.8, 104.6, 103.9, 101.1, 79.99, 33.34, 33.10, 28.54, 18.49
IR (CHCl3) 3400, 2924, 1615, 1423, 1364, 1169, 1105, 1024 cm-1
ESI-MS m/z 327 [M+1]+
HRMS m/z 349.1045 calcd for C19H18O5+Na+ (349.1015)Lead Reference
Other References
Selenski, C.; Pettus, T. R. R. Tetrahedron, 2006, 62, 5298–5307 http://dx.doi.org/10.1016/j.tet.2006.01.109
Roux, D.G. Biochem. J. , 1963, 87, 435-439. http://www.biochemj.org/bj/087/0435/0870435.pdf
Ahluwalia, V. K.; Arora, K. K.; Mukherjee, K. Synthesis, 1984, 4, 346-348. http://dx.doi.org/10.1055/s-1984-30840).
Supplementary Information
Keywords
[4+2]-Diels-Alder cycloaddition, addition, flavans, heterocyclic compounds, O-quinone methide