DCC mediated esterification of 2,4-dinitrophenol
SyntheticPage 705
DOI:
Submitted: November 25, 2013, published: December 3, 2013
Authors
Alexey Medved'ko (lexeym@gmail.com)
Gregory Kazankov (gkazankov@hotmail.com)
Sergey Kurzeev (kurzeev@gmail.com)
Sergey Vatsadze (zurabych@gmail.com)
A contribution from
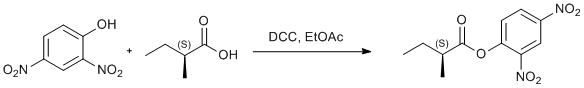
Chemicals
2,4-dinitrophenol (Labteh)
(S)-2-methylbutanoic acid (Sigma)
N,N-dicyclohexylcarbodiimide DCC (Sigma)
Ethyl acetate (Irea)
Petroleum ether 40-60°C (Irea)
Dichloromethane (Irea)
Silica gel
(S)-2-methylbutanoic acid (Sigma)
N,N-dicyclohexylcarbodiimide DCC (Sigma)
Ethyl acetate (Irea)
Petroleum ether 40-60°C (Irea)
Dichloromethane (Irea)
Silica gel
Procedure
2,4-dinitrophenol (0.4484 g, 2.44 mmol) and (S)-2-methylbutanoic acid (0.265 ml, 2.43 mmol) of were dissolved in dry ethyl acetate (4 ml) and cooled in ice bath. DCC (0.5074 g, 2.46 mmol) of was dissolved in dry ethyl acetate (4 ml) and was added to above solution in one portion under vigorous stirring. The reaction mixture was stirred for 1 h with cooling and 19 h at room temperature. The precipitated N,N-dicyclohexylurea was filtered off and the solvent was removed from the filtrate on rotary evaporator. The yellow oil thus obtained was purified with column chromatography. Eluent: petroleum ether:DCM:ethyl acetate 5:1:1. Product obatained as yellow oil that slowly solidifies in refrigerator. Yield 100% (650.1 mg).
Author Comments
2,4-Dinitrophenyl ester elutes as the first yellow band, prior to 2,4-dinitrophenol.
Data
Yellow solid.
[α]d20 +16.6° (c 0.54, EtOAc).
1H-NMR (400MHz, CDCl3, δ, ppm): 1.05 (t, 3H, J 7.4Hz), 1.36 (d, 3H, 3J = 7Hz), 1.66 (dt, 1H, 3J = 14.1Hz, 3J = 7Hz), 1.9 (dt, 1H, 3J = 14.1 Hz, 3J = 7.2Hz), 2.75 (sext, 1H, 3J = 6.9Hz), 7.46 (d, 1H, 3J = 8.9Hz), 8.51 (dd, 1H, 3J = 8.9Hz, 3J = 2.7Hz), 8.94 (d, 1H, 3J = 2.6Hz).
[α]d20 +16.6° (c 0.54, EtOAc).
1H-NMR (400MHz, CDCl3, δ, ppm): 1.05 (t, 3H, J 7.4Hz), 1.36 (d, 3H, 3J = 7Hz), 1.66 (dt, 1H, 3J = 14.1Hz, 3J = 7Hz), 1.9 (dt, 1H, 3J = 14.1 Hz, 3J = 7.2Hz), 2.75 (sext, 1H, 3J = 6.9Hz), 7.46 (d, 1H, 3J = 8.9Hz), 8.51 (dd, 1H, 3J = 8.9Hz, 3J = 2.7Hz), 8.94 (d, 1H, 3J = 2.6Hz).
Lead Reference
V. K. Kibirev, A. A. Gershkovich Sintez peptidov. Reagenty i metody (in Russian) // Kiev, 1987, p. 59.
Keywords
alcohols, carboxylic acids, esters, nucleophilic, substitution
Comments
Racemization
Looks like a useful protocol--thanks. It seems that the major advantage of this method is that racemization can be avoided. I don't see where this has been addressed. Perhaps the optical rotation is enough to make this conclusion. Since the original article is in Russian, it may be useful to establish here that this procedure does not result in any racemization.
By Warren G. Lewis on December 3, 2013