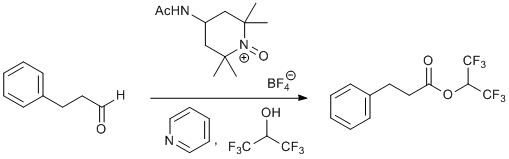
Oxidative Esterification of 3-Phenylpropanal with Hexafluoroisopropanol and an Oxoammonium Salt Oxidant
SyntheticPage 704
DOI:
Submitted: November 20, 2013, published: November 30, 2013
Authors
Christopher Kelly (christopher.b.kelly@uconn.edu)
Michael Mercadante (michael.mercadante@uconn.edu)
Rebecca Wiles (rebecca.wiles@uconn.edu)
A contribution from
Chemicals
3-phenylpropanal (Prepared In-House, See Comment 2)
Hexafluoroisopropanol (99%,Synquest Labs)
Pyridine (anhydrous, 99.8%, Sigma Aldrich)
Procedure
To a one-neck 50 mL round bottom flask equipped with a stir bar was added 3-phenylpropanal (0.671 g, 5 mmol), pyridine3 (5.04 g, 63.75 mmol, 12.75 equiv) and HFIP (2.52 g, 3 equiv). The mixture was allowed to stir at room temperature for approximately five minutes. At this time, the oxoammonium salt (3.75 g, 12.5 mmol, 2.5 equiv) was added all at once4 and the flask was sealed with a rubber septum. The reaction mixture was stirred at room temperature and gradually turned red.5 Once the reaction was judged6 complete , the HFIP was removed in vacuo by rotary evaporation (≈ 15 mmHg, 37 oC water bath7). To this thick residue was added pentane8 (≈ 30 mL) causing immediate precipitation of the spent oxidant, the 4-NHAc-TEMPO radical (often called the "nitroxide"). The heterogeneous solution was allowed to stir for five minutes and the solids were filtered off through a medium porosity fritted funnel washing with pentane (≈ 250 mL). The solids were saved9 and filtrate was transferred to a separatory funnel and washed with 0.5 M HCl twice (≈ 150 mL). The organic layer was washed with deionized water (≈ 150 mL) and brine (≈ 150 mL). The organic layer was dried with Na2SO4 and the solvent was removed in vacuo by rotary evaporation (100 mmHg, 37 oC water bath6) affording the pure ester (1.31 g, 87%) as a clear, yellow oil.
Author Comments
Data
1H NMR (CDCl3, 400 MHz) d ppm 2.92 (t, J=7.40 Hz, 2 H) 3.11 (t, J=7.40 Hz, 2 H) 5.89 (spt, J=6.00 Hz, 1 H) 7.27 - 7.36 (m, 3 H) 7.37 - 7.44 (m, 2 H)
13C NMR (CDCl3, 100 MHz) d ppm 30.17 (CH2) 34.56 (CH2) 66.26 (spt, JC-C-F=34.50 Hz, CH) 120.20 (q, JC-F=282.40 Hz, CF3) 126.45 (CH) 127.92 (CH) 128.43 (CH) 138.92 (C) 169.35 (C)
19F NMR (CDCl3, 377 MHz) -76.25 (d, J=6.81 Hz) (Referenced to Hexafluorobenzene, -164.9 ppm)
GC-MS (EI) 300 ([M]+, 45%) 133 (11%) 105 (34) 104 (80%) 103 (15%) 91 (100%) 77 (19%) 69 (13%) 51 (10%)
HRMS (ESI+) calcd for C12H10F6O2 [M+]: 300.0585, found: 300.0609.
Lead Reference
Other References
Keywords
aldehydes, aromatics/arenes, esters, Green Chemistry, Organic Oxidants, Organofluorine, oxidation, Oxoammonium Salts