O-Alkylation of Salicylaldehyde with Ethyl Bromoacetate and Subsequent Dehydrative Annulation
SyntheticPage 703
DOI:
Submitted: November 20, 2013, published: December 4, 2013
Authors
Christopher Kelly (christopher.b.kelly@uconn.edu)
A contribution from
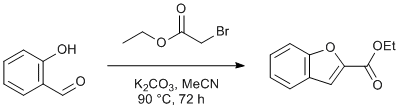
Chemicals
Ethyl Bromoacetate (98%, Sigma-Aldrich)
Potassium Carbonate (99.99%, trace metal basis, Sigma Aldrich)
Acetonitrile (ACS reagent, ≥99.5%, Sigma-Aldrich)
Procedure
Author Comments
1. CAUTION: Powerful lachrymator and highly toxic! Handle with care!
2. A coarse filter funnel is highly recommended as the resulting solids have a tendency to clog finer frits, resulting in very time-consuming filtrations
3. While the ether is removed quiet readily, residual acetonitrile is difficult to remove unless high vacuum is used (> 5 mmHg)
4. Despite the color, the product was pure by NMR spectroscopy and suitable for further reactions. However, if further purity is required, distillation of this compound can be performed under very high vacuum ( > 0.4 mmHg)
Data
1H NMR (CDCl3, 400 MHz) d ppm 1.37 (t, J = 7.09 Hz, 3 H) 4.39 (q, J = 7.09 Hz, 2 H) 7.20 - 7.26 (m, 1 H) 7.37 (ddd, J = 8.44, 7.21, 1.22 Hz, 1 H) 7.46 (d, J = 0.98 Hz, 1 H) 7.53 (dd, J = 8.31, 0.73 Hz, 1 H) 7.58 - 7.63 (m, 1 H)
13C NMR (CDCl3, 100 MHz) d ppm 14.37 (CH3) 61.52 (CH2) 112.36 (CH) 113.80 (CH) 122.85 (CH) 123.81 (CH) 127.03 (CH) 127.61 (C) 145.79 (C) 155.75 (C) 159.60 (C)
GC-MS (EI) 190 ([M]+, 68%) 162 (79%) 145 (100%) 134 (9%) 118 (42%) 89 (68%) 78 (8%) 63 (25%)
Lead Reference
Keywords
Benzofuran, Condensation, Cyclization, esters, heterocyclic compounds, nucleophilic, substitution