Mild esterification with diazomethane
SyntheticPage 701
DOI:
Submitted: November 12, 2013, published: November 13, 2013
Authors
John MacMillan (john.macmillan@temple.edu)
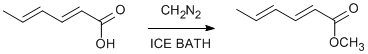
Chemicals
trans,trans-2,4-Hexadienoic (Sorbic) acid (Aldrich, 24,019-9)
Potassium hydroxide, pellets, 85%, A.C.S reagent (Aldrich, 22147-3)
Glacial acetic acid, 99.7%, A.C.S. reagent (Aldrich, 24,285-3)
Ethyl Alcohol, 190 proof, 95+%, A.C.S. spectrophotometric grade (Aldrich, 49,351-1)
Sodium hydrogen carbonate, 99.7%, A.C.S. reagent, Aldrich, 43,144-3
Ether, anhydrous, 99.8%, A.C.S. reagent (Aldrich, 44,354-9)
Procedure
Author Comments
Data
B.p 70o (25mm), Ir (film) 3025(m), 3000(m), 2950(m), 2850(m), 1720 (vs),1645(s),1615(s),1430(s),1370(m).1315(s).1290(m),1260(s),1240(s).1210(m),1185(m),1135(s),1075(m),1025(m),990(s),930(m),915(w), 890(m),850(m),840(m),785(m) and 700(m) cm-1 Pmr (60Mhz), CCl4 δ7.18(m, 1H, Hβ), 6.15(m, 2H, Hγ + Hβ), 5.71(d, 1H, J=16Hz, Hα), 3.66(s,3H, OCH3 ), 1.83(d, J=6Hz, 3H, methyl).
The ester contained ~10%-2E,4Z-isomer. The spectrum becomes fully first order on addition of Eu(fod)3 (fod= 1,1,1,2,2,3,3-heptafluoro-7,7-dimethyl-4,6-octanedionato).
The isomers are fully resolved with lanthanide chemical shift reagents. See other references. Coupling constants for the 2E,4E isomer are: Jαβ = 16 Hz, Jβγ = 10 Hz, Jγ∆ = 15 Hz, J∆-CH3 = 6 Hz.
Lead Reference
Derivatization of Carboxylic Acids with Diazomethane and Trimethylsilyldiazomethane: Convenient Methods and Artifacts
http://userpage.chemie.fu-berlin.de/~tlehmann/krebs/files_diazoalkanes.pdf
Other References
Keywords
addition, carboxylic acids, esters