Palladium mediated displacement of crotyl bromide by an aryllithium
SyntheticPage 70
DOI:
Submitted: August 16, 2001, published: August 17, 2001
Authors
Jonathan Wilden (jonathan_wilden@hotmail.com)
A contribution from
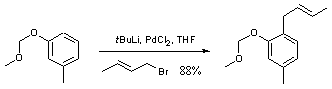
Chemicals
1-Methoxymethoxy-3-methylbenzene,
t-Butyllithium (1.7 M, in pentane, Acros),
Palladium(II) Chloride (Acros)
Crotyl bromide (Aldrich),
Tetrahydrofuran (distilled from sodium / benzophenone ketyl),
Pentane (dried over sodium wire).
t-Butyllithium (1.7 M, in pentane, Acros),
Palladium(II) Chloride (Acros)
Crotyl bromide (Aldrich),
Tetrahydrofuran (distilled from sodium / benzophenone ketyl),
Pentane (dried over sodium wire).
Procedure
1-Methoxymethoxy-3-methylbenzene (0.51 g, 3.3 mmol), was dissolved in pentane (10 mL) under a nitrogen atmosphere, cooled to -78 oC and t-butyllithium (3.0 ml, 4.0 mmol, 1.7 M in pentane) was added with stirring. After 10 minutes the cooling bath was removed and the mixture allowed to warm to ambient temperature, forming a white precipitate. Stirring was then ceased and the precipitate was allowed to settle for 6 hours. The supernatant was removed via syringe and the precipitate dissolved in THF (20 mL). Palladium(II) chloride (30 mg, 0.17 mmol) was added and the solution cooled to -78 oC. Crotyl bromide (0.33 mL, 3.3 mmol) was added dropwise with stirring and the reaction allowed to warm to ambient temperature and stirred for 15 minutes. Water (100 mL) was added and the reaction extracted into ether (3 x 50 mL). The organic fractions were combined, washed with brine (50 mL), dried (MgSO4) and filtered. The filtrate was removed in vacuo and the crude residue was subjected to flash column chromatography (5% Ether / Petrol) to yield the product as a pale yellow oil (0.58 g, 2.9 mmol, 88%).
Author Comments
We performed this reaction twice. In both cases the scale was around 0.5 g. It is essential that the pentane suspension of the organolithium reagent is allowed to settle for at least 5-6 hours if good yields are to be obtained. Care should be taken when decanting the supernatant pentane so as not to disturb the anion precipitate.
Data
1H NMR (300 MHz, CDCl3) 7.07 (1H, d), 6.93 (1H, s), 6.81 (1H, d), 5.55-5.29 (2H, m) 5.10 (2H, s), 3.53 (3H, s), 3.34 (2H, br. d), 2.23 (3H, s), 1.70 (3H, dd).
Lead Reference
Wilden, J. D.; PhD thesis, University of Southampton, 2000.