TBDMS protection of 2,5-hydroxybenzaldehyde
SyntheticPage 661
DOI:
Submitted: July 25, 2013, published: July 29, 2013
Authors
Jan M. Becker (j.m.becker@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
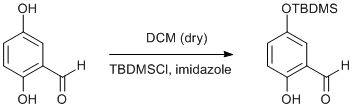
Chemicals
TBDMSCl (Aldrich)
Imidazole (Aldrich)
Dichloromethane (distilled over potassium)
Procedure
To a solution of 2,5-hydroxybenzaldehyde (1.24 g, 8.97 mmol) in dry dichloromethane (20 cm3) were added TBDMSCl (1.35 g , 8.97 mmol) and imidazole. (0.61 g, 8.97 mmol) The mixture was stirred overnight and filtered to remove all insoluble material. All volatiles were removed under reduced pressure and the remaining solid (88% purity by 1H NMR) recrystallised from hot hexanes. (1.40 g, 62%)
Author Comments
Data
1H NMR (400 MHz, CDCl3): δ no ArOH observed, 9.16 (s, 1H, HC=O), 6.85 (dd, 1H, 3J = 8Hz, 4J = 2 Hz, ArH), 6.73 (d, 1H, 4J = 2Hz, ArH), 6.68 (d, 1H, 3J = 8Hz ArH).
Lead Reference
Other References
http://dx.doi.org/10.1016/0040-4039(96)00713-7.
Keywords
addition, aldehydes, alkenes, aromatics/arenes, protection, TBDMSCl