Thionation of amides using Lawessons reagent
SyntheticPage 66
DOI:
Submitted: August 16, 2001, published: August 16, 2001
Authors
Stephen Caddick (s.caddick@sussex.ac.uk)
A contribution from
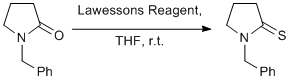
Chemicals
Substrate (2.8 mmoles)
Lawessons reagent (1.4 mmoles)
THF (170 ml)
Lawessons reagent (1.4 mmoles)
THF (170 ml)
Procedure
Dissolve Lawessons reagent (580 mg, 1.43 mmoles) in THF (120 ml) and add THF (50 ml) solution of the amide (500 mg, 2.86 mmoles) to this reagent at room temperature. Stir at room temperature until reaction is complete by tlc (in this case 30 minutes but you can leave the reaction overnight if more convenient). Evaporate solvent and then carry out a standard aqueous work-up using water and extraction with ether. Purification by silica-gl chromatography (petroleum ether: ether gradient, 3:1, 2:1, 1:1) to give the product. Yield 472 mg, 86%.
Author Comments
Lawessons reagent can be really very useful for this transformation. The original procedure used toluene as solvent and the reaction carried out at elevated temperature over prolonged periods. However carrying out the reaction in THF allows it to be conducted at room temperature with reduced reaction times, but it is important that the Lawessons dissolves in the reaction medium and that does take a great deal of THF. It is critical that a good aqueous work-up is used prior to chromatography, do not be tempted to simply remove the solvent and carry out chromatography as impurities will persist. The non-polar product tends to run high up the tlc plate. Note also that Lawessons and impurities are unpleasant smelling species. In my opinion this works better than procedure using P4S10.
Data
1H NMR
7.3, 5H, m, Ph; 5.0, 2H, s, PhCH2; 3.6, 2H, t, CH2N; 3.1, 2H, t, CH2CS; 2.0, 2H, m, CH2;
Lead Reference
Pederson, Lawesson, Bull. Soc. Chim. Belg. 1978, 87, 223, 229 & 293. See also Bull. Soc. Chim. Belg. 1981, 90, 103
Keywords
Comments
What are your suggestions for a good aqueous workup as most papers just remove the solvent followed by column chromatography?
By Helen Rachel lagiakos on April 26, 2007
Thionation
If you just remove solvent the chromatography does not work - you must wash with copious water to remove by products from Lawessons.
By Stephen Caddick on December 6, 2013
thionation reactions
what the difference between Lawessons reagent and
P4S10 as thionating agents??
By Elsayed Mohamed Elsayed Ahmed on December 5, 2013
Thionation
In practical terms you can use THF at room temperature using Lawessons - but you must make sure it is fuly dissolved in THF. From memory this takes a very large volume of THF
By Stephen Caddick on December 6, 2013