Imination of 4-aminopyridine with salicylaldehyde
SyntheticPage 649
DOI:
Submitted: July 23, 2013, published: July 24, 2013
Authors
Lihong Li (Lihongli0707@yahoo.com)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
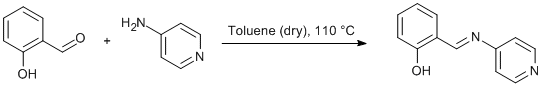
Chemicals
4-aminopyridine (Alfa Aesar)
Toluene (distilled over sodium)
Procedure
A 250 mL round-bottom flask fitted with a PTFE stopcock sidearm was charged with a suspension of salicylaldehyde (2.440 g, 20.0 mmol) and 4-aminopyridine (0.940 g, 10 mmol) in dry toluene (200 mL). A Soxhlet extractor equipped with a dry paper thimble charged with excess powdered CaH2 and condenser was fitted. The condenser outlet was fitted with a T-joint allowing the reaction to be performed under an atmosphere of dry dinitrogen. The system was heated to reflux for 3 d. Toluene was removed to ca. 50 mL in vacuo under Schlenk conditions and the solution was left overnight. The resulting yellow precipitate was isolated by filtration (1.50 g, 75 %) and handled only under a dry atmosphere.
Author Comments
Normal condensation reaction is unsuccesful due to NH2 group being poorly nucleophilic, hence the use of CaH2 to remove water. Hyrdrolysis of this Schiff base is facile.
Data
1H NMR (400MHz, CD3CN): ö12.70 (s, 1H, Ar-OH), 8.85 (s, 1H, CH=N), 8.65 (m, 2H, Py-CH), 7.60 (dd, 3J= 7.6 Hz, 4J = 1.6 Hz, 1H, ArH), 7.49 (m, 1H, ArH), 7.30 (m, 2H, Py-CH), 7.03 (m, 2H, ArH).
13C{1H}NMR (100 MHz, CDCl3): δ 166.9 (CH=N), 160.8 (Ar-Cq), 155.0 (PyCq), 150.74 (Py-CH), 133.9 (Ar-CH), 133.1 (Ar-CH), 119.1 (Ar-Cq), 117.0 (ArCq), 116.6 (Ar-CH), 115.9 (Py-CH).
MS(ESI) m/z 199.1 [M+H]+
Anal. Found (Calculated for C12H10N2O): C 72.76 (72.71), H 5.23 (5.08), N 14.13 (14.13) %.
Lead Reference
http://dx.doi.org/10.1002/chem.200801932
Other References
DOI: 10.1039/C1CC15574A
Keywords
addition, alcohols, aldehydes, amination, amines, aromatics/arenes, Schiff base