Acetylation of hydroxypyranones
SyntheticPage 64
DOI:
Submitted: August 15, 2001, published: August 16, 2001
Authors
lisa frost (lisa.frost@evotec.com)
A contribution from
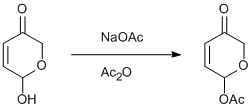
Chemicals
6-hydroxy-2,3-dihydro-6H-pyrano-3-one
acetic anhydride (Avocado)
sodium acetate (Avocado)
acetic anhydride (Avocado)
sodium acetate (Avocado)
Procedure
To a cold solution (0 oC) of 6-hydroxy-2,3-dihydro-6H-pyrano-3-one (65.3 g, 0.57 mol) in THF (600 mL) was added acetic anhydride (234 g, 2.29 mol) and sodium acetate (188 g, 2.29 mol). The slurry was stirred for 18 hours at ambient temperature, following which, the resulting orange solution was filtered under pressure to remove any residual solid and concentrated in vacuo. The reaction was quenched by addition of saturated sodium bicarbonate solution (1 L) and additional solid sodium hydrogen carbonate until effervescence ceased. The organic portions were extracted into diethyl ether (2x1 L), dried over MgSO4, filtered and concentrated in vacuo (cold bath) to yield an orange oil. Purification of this oil was achieved by column chromatography on silica gel with petrol: diethyl ether (1:4 v:v) as the eluent to give the required product as a yellow oil (59.6 g, 67 %)
Author Comments
This reaction has been carried out routinely in our lab for a number of years by both undergraduates and postgraduates / postdoctorals . Although it is usually carried out on a large scale (70 g) the method is robust and can be utilised equally as well on a small scale. One drawback to this methodology is the work up procedure. The reaction calls for 4 equivalents of sodium acetate and acetic anyhdride. Removal of this excess and the acetic acid formed in the reaction means that the amount of aqueous phase collected is large and hence you find yourself having to deal with large scale extractions. e.g. a 70g scale reaction usually requires somewhere in the 5L scale of sodium bicarbonate solution to quench the reaction. One way to reduce this is to remove any solid before quenching. As with all other reactions in this sequence it is wise to wear gloves during handling at all stages.
This transformation has also been attempted using acetyl chloride and pyridine. This method works equally as well on a small scale but removal of pyridine on a large scale is not desirable.
Data
1H nmr (300 MHz, CDCl3) 2.15 (3H, s), 4.23 (1H, d J=1.7Hz), 4.51 (1H, d J=17Hz), 6.18 (1H, d J=11Hz), 6.47 (1H, d J=3.6Hz), 6.95 (1H, dd J=11, 3.6Hz); 13C nmr (75 MHz, CDCl3) 21.3, 67.8, 87.0, 129.2, 142.7, 169.9, 193.8.
Lead Reference
Caddick S, Khan S, Frost LM, Smith NJ, Cheung S, Pairaudeau G; Tetrahedron, 2000, 56, 8953-8958.