Difluorination of alpha hydroxy ketones
SyntheticPage 63
DOI:
Submitted: August 14, 2001, published: August 16, 2001
Authors
lisa frost (lisa.frost@evotec.com)
A contribution from
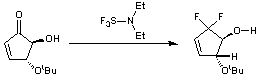
Chemicals
5-tert-butyl-4-hydroxycyclopent-2-one
Diethylaminosulfur trifluoride (DAST)
DCM (distilled from calcium hydride)
Diethylaminosulfur trifluoride (DAST)
DCM (distilled from calcium hydride)
Procedure
To a solution of 5-tert-butyl-4-hydroxycyclopent-2-one (200 mg, 1.2 mmol) in DCM (4 mL) cooled to -78 oC was added diethylaminosulfur trifluoride (948 mg, 5.9 mmol). The reaction was slowly allowed to warm to an ambient temperature over 4 h and stirring was continued for a further 24 h. The reaction mixture was poured onto basic alumina, washed with water (5 mL), dried (Na2SO4), filtered and concentrated in vacuo. Purification was achieved by chromatography on silica gel with petrol:ethyl acetate (10:1 v:v) as the eluent, yielding the title compound as colourless crystals (224 mg, 98 %).
Author Comments
This reaction is very high yielding if 5 equivalents of DAST is employed. Unfortunately, when the equivalents are reduced the yield of the reaction falls to around 30 %. The reaction should be carried out in plastic vessels as DAST etches glass. Care must be taken when pouring the reaction mixture onto alumina as there is a vigorous reaction. This limits the scale of the reaction. In the four times I have carried out this reaction I have only prepared 100mg quantities of product. Further the quality of the DAST is important. In our hands the DAST from Aldrich was optimal. As you can imagine this reaction cannot be carried out on molecules containing silicon groups as they are readily removed under these conditions
Data
1H nmr(500MHz, CDCl3) 1.21 (9H, s), 2.39 (1H, br s), 4.08 (1H, ddd, J=4.4, 8.6, 12.9 Hz), 4.43 (1H, dddd, J=1.5, 3.2, 4.6, 9.0 Hz), 5.86 (1H, ddd, J=1.4, 1.4, 6.4 Hz), 6.09 (1H, dddd, J=0.7, 1.6, 1.6, 6.4 Hz); 19F nmr (282 MHz, CDCl3) –106.35 (ddd, J=255.4, 8.1, 3.2 Hz), -95.72 (dddd, J=255.4, 12.6, 7.5, 1.6Hz); 13C nmr (75MHz, CDCl3) 28.2, 75.2, 78.4 (dd, J=1.4, 7.1 Hz), 80.9 (t, J=23.1 Hz), 125.9 (dd, J=248.2, 241.6 Hz), 126.9 (dd, J=22.5, 30.6 Hz), 143.9 (dd, J=8.2, 10.9 Hz)
Lead Reference
Caddick S, Cheung S, Doyle VE, Frost LM, Soscia MG, Delisser VM, Williams MRV, Etheridge ZC, Khan S, Hitchcock PB, Pairaudeau G, Vile S
Tetrahedron, 57, 6295-6303
Other References
Wilkinson, J. A.; Chem. Rev., 1992, 505; Hudlicky, M.; Org. React. (New York), 1988, 35, 515