BOC deprotection of an aminopropyl methanesulfonate
SyntheticPage 616
DOI:
Submitted: July 11, 2013, published: July 15, 2013
Authors
Nikola P. Chmel (N.Chmel@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
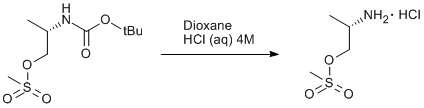
Chemicals
Dioxane (Fischer Scientific)
HCl (aq) 4M
Procedure
A solution of (S)-2-(tert-butoxycarbonylamino)propyl methanesulfonate (2.0 g, 7.9 mmol) in dioxane (10 ml) was acidified with an aqueous solution of HCl (10 ml, 4 M) and was stirred for 1 h. The volatiles were removed under reduced pressure and the residue was washed with acetonitrile. Yield 0.84 g (56%).
Author Comments
Data
1H NMR (400 MHz, 298 K, MeOD) δH 4.44 (1H, dd, 3JHH = 11.2 Hz, 2JHH = 3.4 Hz, CH2), 4.27 (1H, dd, 3JHH = 11.2 Hz, 2JHH = 6.8 Hz, CH2), 3.73 – 3.62 (1H, m, CH), 3.19 (3H, s, SO3CH3), 1.37 (3H, d, 3JHH = 6.8 Hz, CHCH3).
13C NMR (100 MHz, 298 K, MeOD) δC 70.65 (CH2), 47.93 (CH), 37.40 (SO3CH3), 15.06 (CH3).
MS (ESI+) m/z 154.2 ([M-Cl]+)
IR (cm-1) ν 2865, 2779, 2713, 2693, 2604, 2525, 2022, 1609, 1508, 1460, 1403, 1387, 1324, 1282, 1211, 1170, 1134, 1022, 998, 967, 947, 928, 852, 819, 769.
Lead Reference
K. Higashiura, H. Morino, H. Matsuura, Y. Toyomaki and K. Ienaga, J. Chem. Soc., Perkin Trans. 1, 1989, 1479-1481.http://dx.doi.org/10.1039/p19890001479
Supplementary Information
Keywords
alcohols, amines, BOC, deprotection, esters, hydrochloride